| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345147 | 1500346 | 2014 | 5 صفحه PDF | دانلود رایگان |
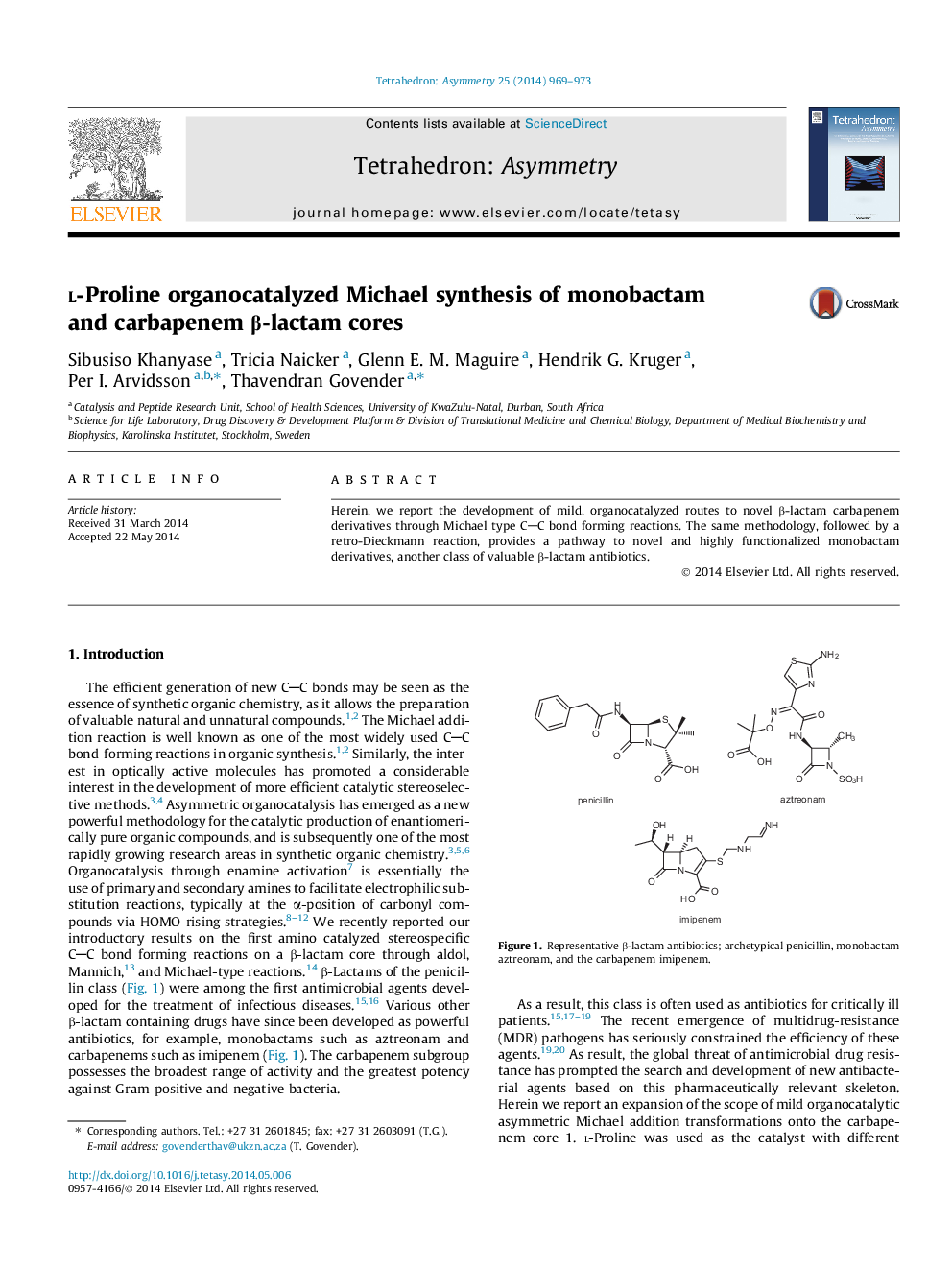
Herein, we report the development of mild, organocatalyzed routes to novel β-lactam carbapenem derivatives through Michael type CC bond forming reactions. The same methodology, followed by a retro-Dieckmann reaction, provides a pathway to novel and highly functionalized monobactam derivatives, another class of valuable β-lactam antibiotics.
Figure optionsDownload as PowerPoint slide
(2S,5R,6S)-4-Nitrobenzyl 6-((R)-1-hydroxyethyl)-2-((S)-1-(4-methoxyphenyl)-2-nitroethyl)-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylateC25H25N3O10[α]D20 = +156.7 (c 0.10 CHCl3)Source of chirality: 4-nitrobenzyl (5R,6S)-6-[(1R)-1-hydroxyethyl]-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylateAbsolute configuration: (2S,5R,6S)(R)(S)
(2S,5R,6S)-4-Nitrobenzyl 2-((R)-1-(4-chlorophenyl)-2-nitroethyl)-6-((R)-1-hydroxyethyl)-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylateC24H22N3O9Cl[α]D20 = +101.0 (c 0.10, CHCl3)Source of chirality: 4-nitrobenzyl (5R,6S)-6-[(1R)-1-hydroxyethyl]-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylateAbsolute configuration: (2S,5R,6S)(R)(R)
(2S,5R,6S)-4-Nitrobenzyl 6-((R)-1-hydroxyethyl)-3,7-dioxo-2-((R)-3-oxocyclopentyl)-1-azabicyclo[3.2.0]heptane-2-carboxylateC21H22N2O8[α]D20 = +215.0 (c 0.10, CHCl3)Source of chirality: 4-nitrobenzyl (5R,6S)-6-[(1R)-1-hydroxyethyl]-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylateAbsolute configuration: (2S,5R,6S)(R)(R)
(2S,5R,6S)-4-Nitrobenzyl 6-((R)-1-hydroxyethyl)-3,7-dioxo-2-((R)-3-oxocyclohexyl)-1-azabicyclo[3.2.0]heptane-2-carboxylateC22H24N2O8[α]D20 = +43.2 (c 0.10, CHCl3)Source of chirality: 4-nitrobenzyl (5R,6S)-6-[(1R)-1-hydroxyethyl]-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylateAbsolute configuration: (2S,5R,6S)(R)(R)
(2S,5R,6S)-4-Nitrobenzyl 6-((R)-1-hydroxyethyl)-3,7-dioxo-2-((S)-3-oxo-1-phenylbutyl)-1-azabicyclo[3.2.0]heptane-2-carboxylateC26H26N2O8[α]D20 = +80.0 (c 0.10, CHCl3)Source of chirality: 4-nitrobenzyl (5R,6S)-6-[(1R)-1-hydroxyethyl]-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylateAbsolute configuration: (2S,5R,6S)(R)(S)
(2S,5R,6S)-4-Nitrobenzyl 6-((R)-1-hydroxyethyl)-2-((S)-4-methoxy-3,4-dioxo-1-phenylbutyl)-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylateC27H26N2O10[α]D20 = +40.1 (c 0.10, CHCl3)Source of chirality: 4-nitrobenzyl (5R,6S)-6-[(1R)-1-hydroxyethyl]-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylateAbsolute configuration: (2S,5R,6S)(R)(S)
(2S,5R,6S)-4-Nitrobenzyl 2-((S)-1-(benzo[d][1,3]dioxol-5-yl)-4-methoxy-3,4-dioxobutyl)-6-((R)-1-hydroxyethyl)-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylateC28H26N2O12[α]D20 = +97.3 (c 0.10, CHCl3)Source of chirality: 4-nitrobenzyl (5R,6S)-6-[(1R)-1-hydroxyethyl]-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylateAbsolute configuration: (2S,5R,6S)(S)(R)
(2S,5R,6S)-4-Nitrobenzyl 2-((S)-1-(4-fluorophenyl)-4-methoxy-3,4-dioxobutyl)-6-((R)-1-hydroxyethyl)-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylateC27H25N2O10F[α]D20 = +50.2 (c 0.10, CHCl3)Source of chirality: 4-nitrobenzyl (5R,6S)-6-[(1R)-1-hydroxyethyl]-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylateAbsolute configuration: (2S,5R,6S)(S)(R)
2-((2R,3S)-3-(1-Hydroxyethyl)-1-(2-(4-nitrobenzyloxy)-2-oxo-1-(3-oxocyclopentyl)ethyl)-4-oxoazetidin-2-yl)acetic acidC21H24N2O9[α]D20 = −17.4 (c 0.10, CHCl3)Source of chirality: 4-nitrobenzyl (5R,6S)-6-[(1R)-1-hydroxyethyl]-3,7-dioxo-1-azabicyclo[3.2.0]heptane-2-carboxylateAbsolute configuration: (2R,3S)
Journal: Tetrahedron: Asymmetry - Volume 25, Issues 13–14, 31 July 2014, Pages 969–973