| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345171 | 980184 | 2007 | 8 صفحه PDF | دانلود رایگان |
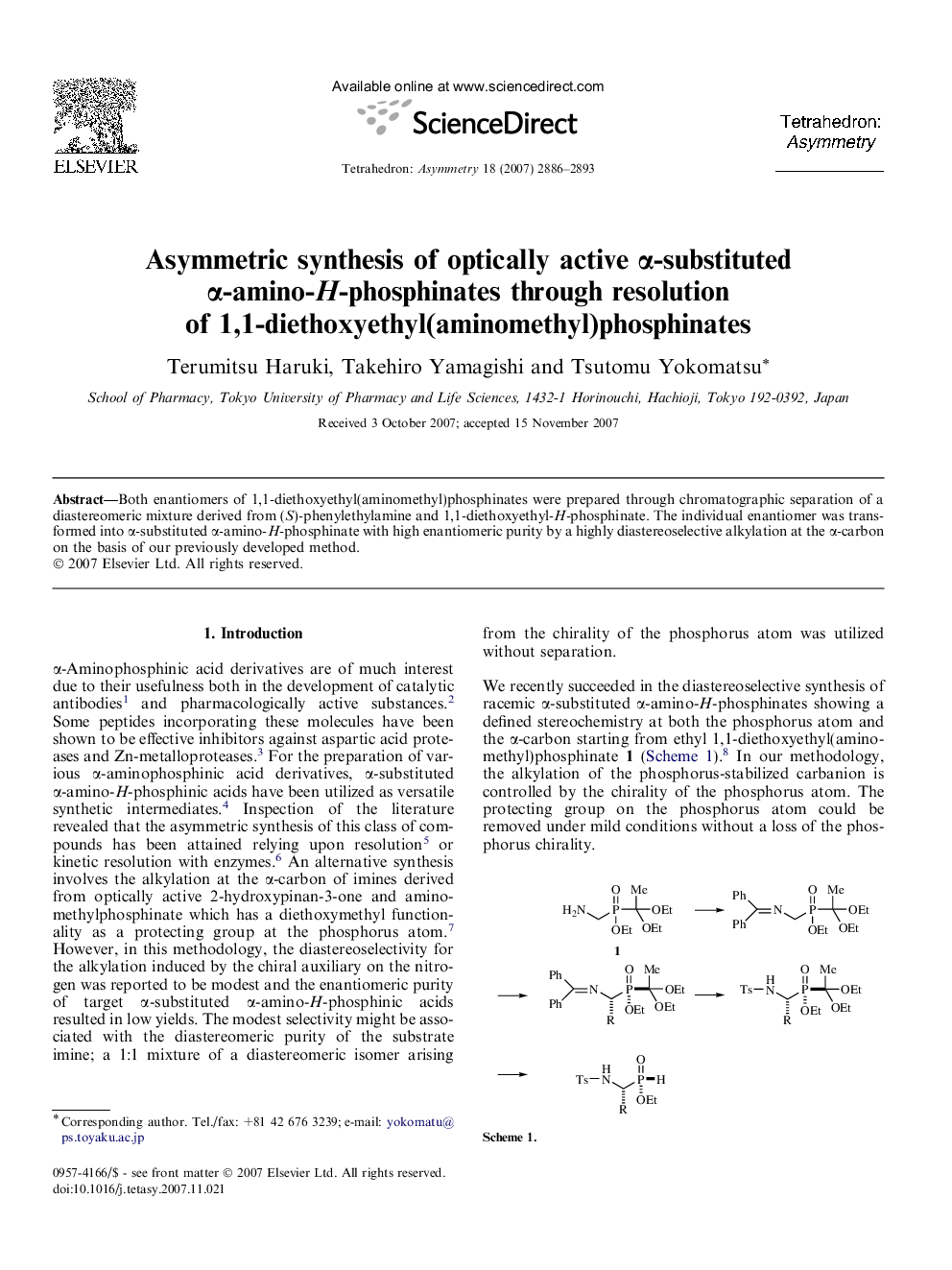
Both enantiomers of 1,1-diethoxyethyl(aminomethyl)phosphinates were prepared through chromatographic separation of a diastereomeric mixture derived from (S)-phenylethylamine and 1,1-diethoxyethyl-H-phosphinate. The individual enantiomer was transformed into α-substituted α-amino-H-phosphinate with high enantiomeric purity by a highly diastereoselective alkylation at the α-carbon on the basis of our previously developed method.
Figure optionsDownload as PowerPoint slide
(SP)-Ethyl 1,1-diethoxyethyl{[(diphenylmethylene)amino]methyl}phosphinateC22H30NO4PEe >99%[α]D24=+16.6 (c 0.6, CHCl3)Source of chirality: (S)-phenylethylamineAbsolute configuration: (SP)
(1S,SP)-Ethyl 1,1-diethoxyethyl{1-[(diphenylmethylene)amino]-2-phenylethyl}phosphinateC29H36NO4PEe >99%[α]D24=+83.5 (c 0.1, CHCl3)Source of chirality: (S)-phenylethylamineAbsolute configuration: (1S,SP)
(1S,SP)-Ethyl 1,1-diethoxyethyl{1-[(diphenylmethylene)amino]-3-methylbutyl}phosphinateC26H38NO4PEe >99%[α]D24=-13.8 (c 0.2, CHCl3)Source of chirality: (S)-phenylethylamineAbsolute configuration: (1S,SP)
(1S,RP)-Ethyl 1-{[(4-methylphenyl)sulfonyl]amino}-2-phenylethylphosphinateC17H22NO4PSEe >99%[α]D24=+69.2 (c 0.08, CHCl3)Source of chirality: (S)-phenylethylamineAbsolute configuration: (1S,RP)
(1S,RP)-Ethyl 1-{[(4-methylphenyl)sulfonyl]amino}-3-methylbutylphosphinateC14H24NO4PSEe >99%[α]D24=+50.5 (c 0.16, CHCl3)Source of chirality: (S)-phenylethylamineAbsolute configuration: (1S,RP)
Journal: Tetrahedron: Asymmetry - Volume 18, Issue 24, 10 December 2007, Pages 2886–2893