| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345223 | 980186 | 2015 | 7 صفحه PDF | دانلود رایگان |
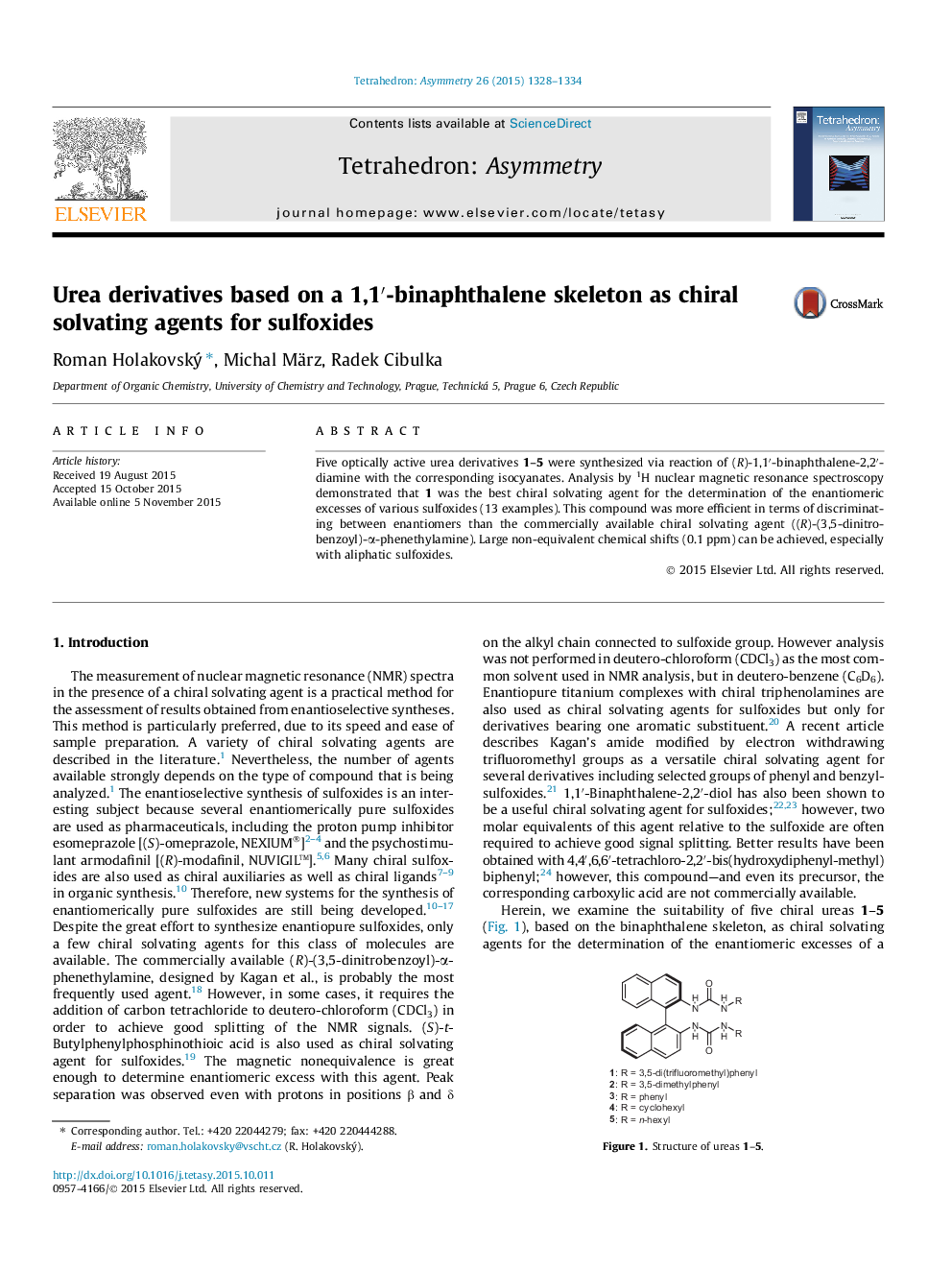
Five optically active urea derivatives 1–5 were synthesized via reaction of (R)-1,1′-binaphthalene-2,2′-diamine with the corresponding isocyanates. Analysis by 1H nuclear magnetic resonance spectroscopy demonstrated that 1 was the best chiral solvating agent for the determination of the enantiomeric excesses of various sulfoxides (13 examples). This compound was more efficient in terms of discriminating between enantiomers than the commercially available chiral solvating agent ((R)-(3,5-dinitro-benzoyl)-α-phenethylamine). Large non-equivalent chemical shifts (0.1 ppm) can be achieved, especially with aliphatic sulfoxides.
Figure optionsDownload as PowerPoint slide
N,N″-(1R)-[1,1′-Binaphthalene]-2,2′-diylbis[N′-[3,5-bis(trifluoromethyl)phenyl]-ureaC38H22F12N4O2[α]D20 = +127.5 (c 0.5, CHCl3)Absolute configuration: (R)
N,N″-(1R)-[1,1′-Binaphthalene]-2,2′-diylbis[N′-3,5-dimethylphenyl]-ureaC38H34N4O2[α]D20 = +57.1 (c 0.5, CHCl3)Absolute configuration: (R)
N,N″-(1R)-[1,1′-Binaphthalene]-2,2′-diylbis(N′-phenyl)-ureaC34H26N4O2[α]D20 = +65.7 (c 0.5, CHCl3)Absolute configuration: (R)
N,N″-(1R)-[1,1′-Binaphthalene]-2,2′-diylbis(N′-cyclohexyl)-ureaC34H38N4O2[α]D20 = +34.7 (c 0.5, CHCl3)Absolute configuration: (R)
N,N″-(1R)-[1,1′-Binaphthalene]-2,2′-diylbis(N′-hexyl)-ureaC34H42N4O2[α]D20 = +31.9 (c 0.5, CHCl3)Absolute configuration: (R)
Journal: Tetrahedron: Asymmetry - Volume 26, Issue 23, 15 December 2015, Pages 1328–1334