| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345230 | 980186 | 2015 | 7 صفحه PDF | دانلود رایگان |
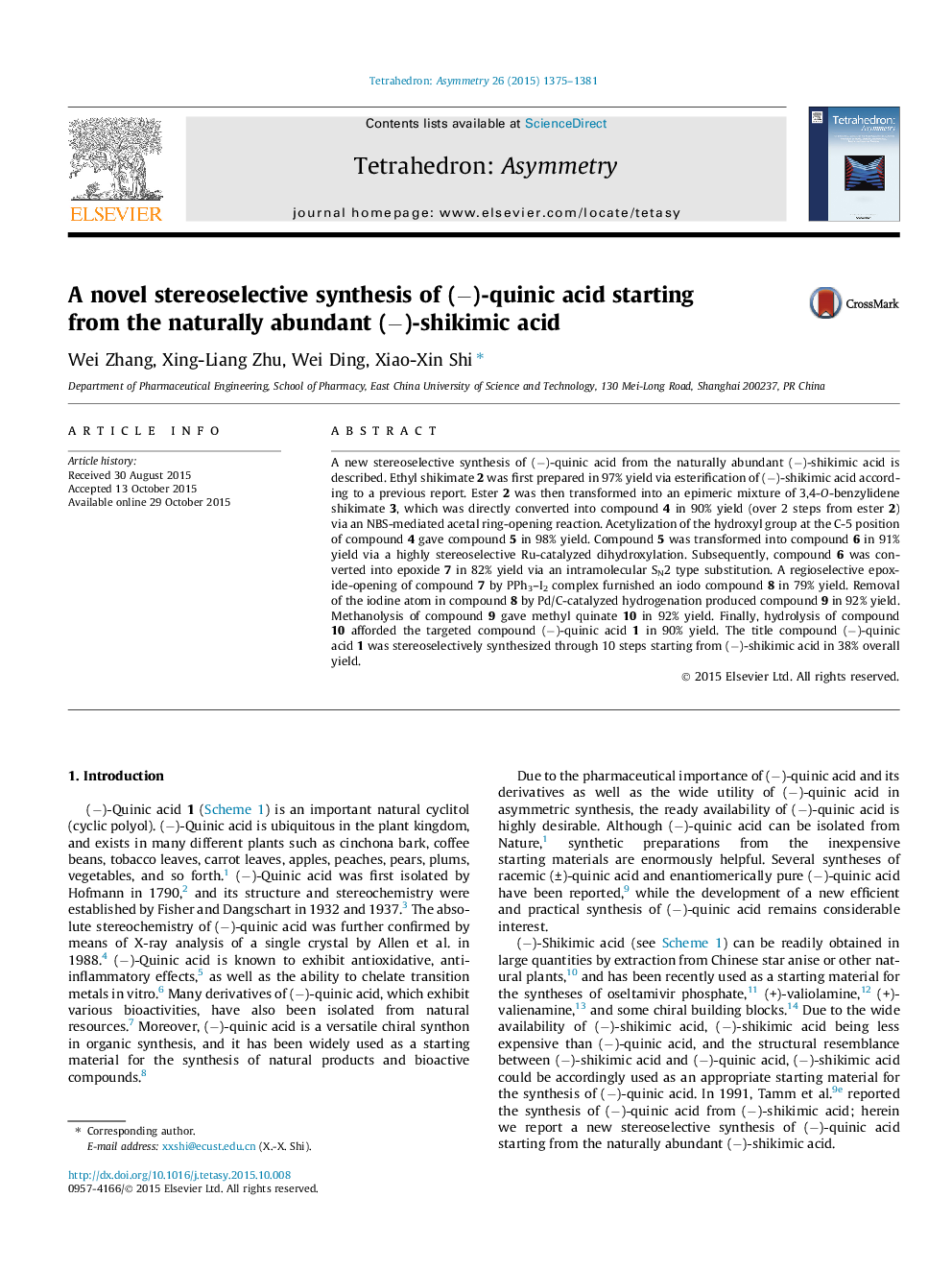
A new stereoselective synthesis of (−)-quinic acid from the naturally abundant (−)-shikimic acid is described. Ethyl shikimate 2 was first prepared in 97% yield via esterification of (−)-shikimic acid according to a previous report. Ester 2 was then transformed into an epimeric mixture of 3,4-O-benzylidene shikimate 3, which was directly converted into compound 4 in 90% yield (over 2 steps from ester 2) via an NBS-mediated acetal ring-opening reaction. Acetylization of the hydroxyl group at the C-5 position of compound 4 gave compound 5 in 98% yield. Compound 5 was transformed into compound 6 in 91% yield via a highly stereoselective Ru-catalyzed dihydroxylation. Subsequently, compound 6 was converted into epoxide 7 in 82% yield via an intramolecular SN2 type substitution. A regioselective epoxide-opening of compound 7 by PPh3–I2 complex furnished an iodo compound 8 in 79% yield. Removal of the iodine atom in compound 8 by Pd/C-catalyzed hydrogenation produced compound 9 in 92% yield. Methanolysis of compound 9 gave methyl quinate 10 in 92% yield. Finally, hydrolysis of compound 10 afforded the targeted compound (−)-quinic acid 1 in 90% yield. The title compound (−)-quinic acid 1 was stereoselectively synthesized through 10 steps starting from (−)-shikimic acid in 38% overall yield.
Figure optionsDownload as PowerPoint slide
(1S,3R,4S,5R)-1,3,4,5-Tetrahydroxycyclohexane carboxylic acid [(−)-quinic acid]C7H12O6[α]D25 = −43.2 (c 1.80, H2O)Source of chirality: (−)-Shikimic acidAbsolute configuration: (1S,3R,4S,5R)
(3S,4S,5R)-Ethyl 3-bromo-4-benzoyloxy-5-hydroxyl-cyclohex-1-ene carboxylateC16H17BrO5[α]D25 = +58.8 (c 1.32, CHCl3)Source of chirality: (−)-Shikimic acidAbsolute configuration: (3S,4S,5R)
(3S,4S,5R)-Ethyl 5-acetoxy-3-bromo-4-benzoyloxy-cyclohex-1-ene carboxylateC18H19BrO6[α]D25 = +87.6 (c 1.30, CHCl3)Source of chirality: (−)-Shikimic acidAbsolute configuration: (3S,4S,5R)
(1R,2R,3S,4S,5R)-Ethyl 5-acetoxy-4-benzoyloxy-3-bromo-1,2-dihydroxy-cyclohexane carboxylateC18H21BrO8[α]D25 = −11.6 (c 2.11, CHCl3)Source of chirality: (−)-Shikimic acidAbsolute configuration: (1R,2R,3S,4S,5R)
(1R,2S,3S,4S,5R)-Ethyl 5-acetoxy-4-benzoyloxy-2,3-epoxy-1-hydroxy-cyclohexane carboxylateC18H20O8[α]D25 = −125 (c 1.62, CHCl3)Source of chirality: (−)-Shikimic acidAbsolute configuration: (1R,2S,3S,4S,5R)
(1S,2R,3S,4R,5R)-Ethyl 5-acetoxy-4-benzoyloxy-1,3-dihydroxy-2-iodo-cyclohexane carboxylateC18H21IO8[α]D25 = −19.6 (c 1.72, CHCl3)Source of chirality: (−)-Shikimic acidAbsolute configuration: (1S,2R,3S,4R,5R)
(1R,2R,3S,4S,5R)-Ethyl 5-acetoxy-4-benzoyloxy-1,2-dihydroxy-3-iodo-cyclohexane carboxylateC18H21IO8[α]D25 = −6.5 (c 1.50, CHCl3)Source of chirality: (−)-Shikimic acidAbsolute configuration: (1R,2R,3S,4S,5R)
(1S,3R,4R,5R)-Ethyl 5-acetoxy-4-benzoyloxy-1,3-dihydroxy-cyclohexane carboxylateC18H22O8[α]D25 = −93.6 (c 1.51, CHCl3)Source of chirality: (−)-Shikimic acidAbsolute configuration: (1S,3R,4R,5R)
(1S,3R,4S,5R)-Methyl 1,3,4,5-tetrahydroxycyclohexane carboxylateC8H14O6[α]D25 = −31.2 (c 1.60, CH3OH)Source of chirality: (−)-Shikimic acidAbsolute configuration: (1S,3R,4S,5R)
Journal: Tetrahedron: Asymmetry - Volume 26, Issue 23, 15 December 2015, Pages 1375–1381