| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345239 | 1500347 | 2014 | 5 صفحه PDF | دانلود رایگان |
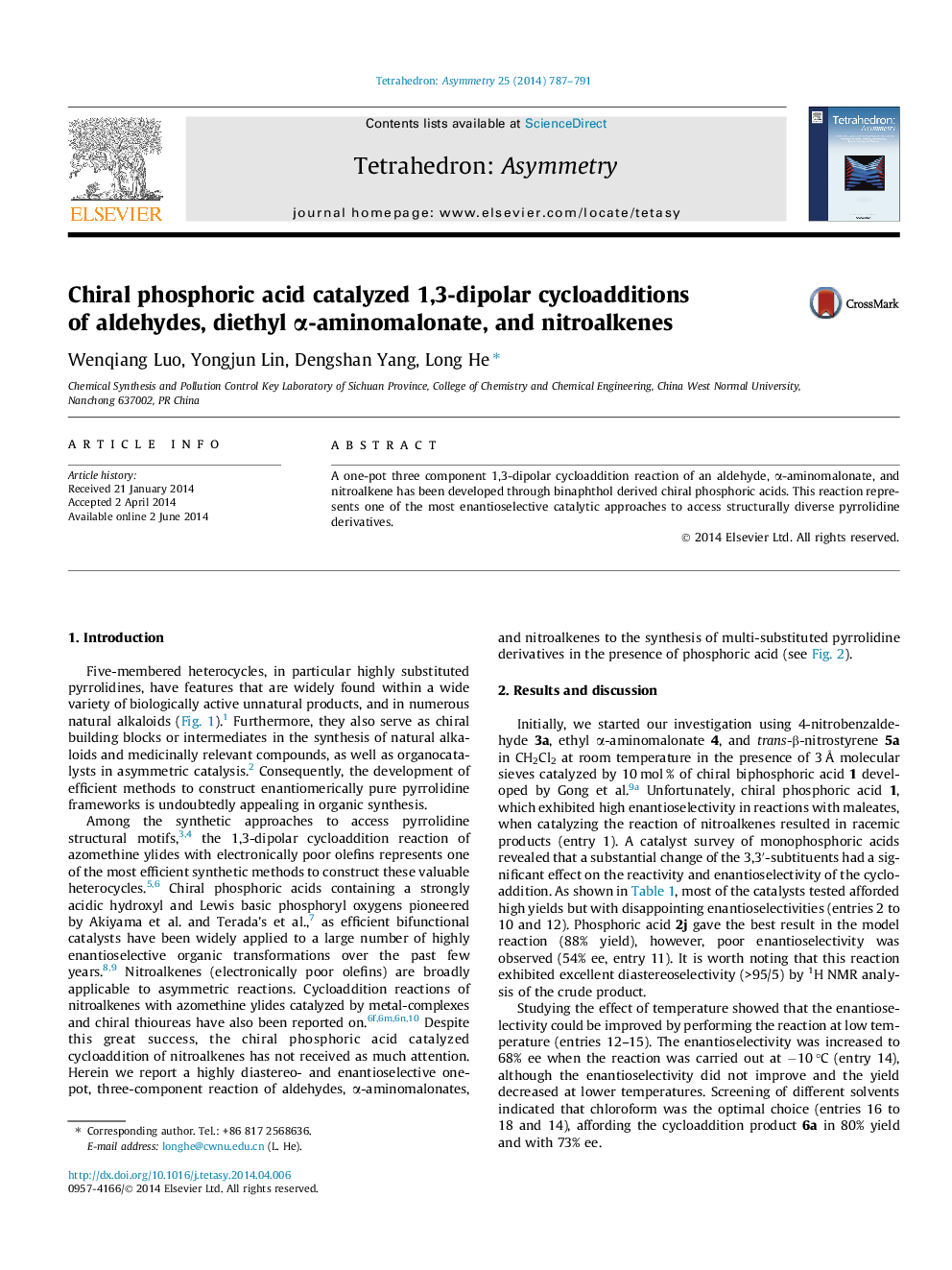
A one-pot three component 1,3-dipolar cycloaddition reaction of an aldehyde, α-aminomalonate, and nitroalkene has been developed through binaphthol derived chiral phosphoric acids. This reaction represents one of the most enantioselective catalytic approaches to access structurally diverse pyrrolidine derivatives.
Figure optionsDownload as PowerPoint slide
Diethyl (3R,4R,5R)-4-nitro-5-(4-nitrophenyl)-3-phenylpyrrolidine-2,2-dicarboxylateC22H23N3O880% yield, 73% ee[α]D20 = +29.3 (c 0.43, CH2Cl2)
3-(4-Bromophenyl)-4-nitro-5-(4-nitrophenyl)pyrrolidine-2,2-dicarboxylateC22H22BrN3O884% yield, 77% ee[α]D20 = +55.4 (c 0.39, CH2Cl2)
Diethyl 4-nitro-3-(3-nitrophenyl)-5-(4-nitrophenyl)pyrrolidine-2,2-dicarboxylateC22H22N4O1090% yield, 65% ee[α]D20 = +47.1 (c 0.87, CH2Cl2)
Diethyl 3-(4-chlorophenyl)-4-nitro-5-(4-nitrophenyl)pyrrolidine-2,2-dicarboxylateC22H22ClN3O885% yield, 70% ee[α]D20 = +27.8 (c 0.78, CH2Cl2)
Diethyl 3-(4-cyanophenyl)-4-nitro-5-(4-nitrophenyl)pyrrolidine-2,2-dicarboxylateC23H22N4O881% yield, 74% ee[α]D20 = +61.4 (c 0.7, CH2Cl2)
Diethyl 3-(4-methoxyphenyl)-4-nitro-5-(4-nitrophenyl)pyrrolidine-2,2-dicarboxylateC23H25N3O971% yield, 70% ee[α]D20 = +46.4 (c 0.7, CH2Cl2)
Diethyl 3-(3-bromophenyl)-4-nitro-5-(4-nitrophenyl)pyrrolidine-2,2-dicarboxylateC22H22BrN3O874% yield, 56% ee[α]D20 = +24.5 (c 0.4, CH2Cl2)
Diethyl 3-(3-chlorophenyl)-4-nitro-5-(4-nitrophenyl)pyrrolidine-2,2-dicarboxylateC22H22ClN3O871% yield, 57% ee[α]D20 = +25.9 (c 0.66, CH2Cl2)
Diethyl 5-(4-bromophenyl)-4-nitro-3-phenylpyrrolidine-2,2-dicarboxylateC22H23BrN2O692% yield, 62% ee[α]D20 = +24.1 (c 0.86, CH2Cl2)
Diethyl 3,5-bis(4-bromophenyl)-4-nitropyrrolidine-2,2-dicarboxylateC22H22Br2N2O688% yield, 70% ee[α]D20 = +43.4 (c 1.0, CH2Cl2)
Diethyl 5-(4-chlorophenyl)-4-nitro-3-phenylpyrrolidine-2,2-dicarboxylateC22H23ClN2O690% yield, 53% ee[α]D20 = +19.3 (c 0.76, CH2Cl2)
Diethyl 3-(4-bromophenyl)-5-(4-cyanophenyl)-4-nitropyrrolidine-2,2-dicarboxylateC23H22BrN3O686% yield, 84% ee[α]D20 = +59.3 (c 0.82, CH2Cl2)
Diethyl 5-(4-cyanophenyl)-4-nitro-3-phenylpyrrolidine-2,2-dicarboxylateC23H23N3O676% yield, 74% ee[α]D20 = +28.6 (c 0.67, CH2Cl2)
Diethyl-4-nitro-3,5-diphenylpyrrolidine-2,2-dicarboxylateC22H24N2O642% yield, 23% ee[α]D20 = +26.6 (c 0.43, CH2Cl2)
Journal: Tetrahedron: Asymmetry - Volume 25, Issues 10–11, 31 May 2014, Pages 787–791