| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345241 | 1500347 | 2014 | 6 صفحه PDF | دانلود رایگان |
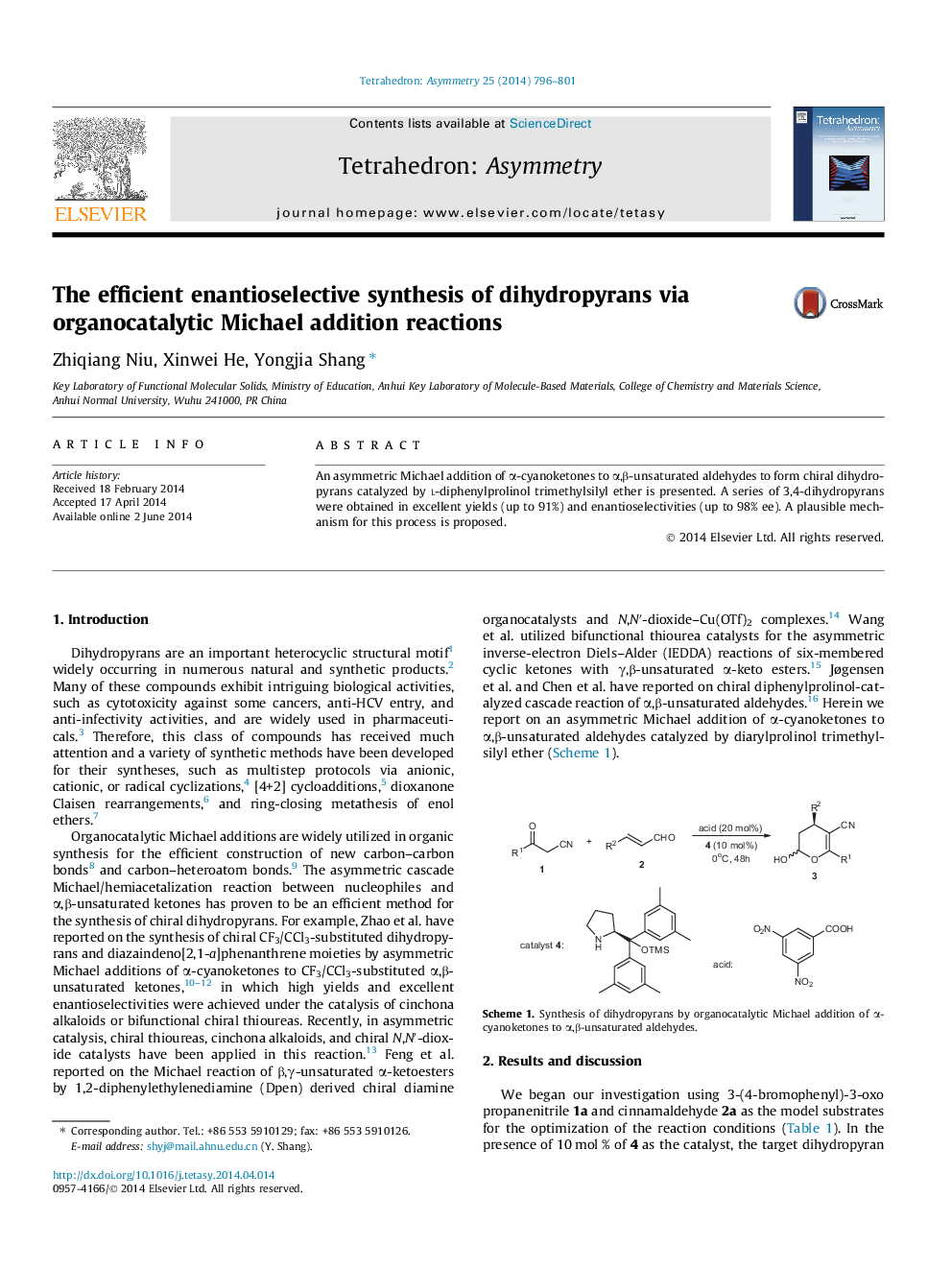
An asymmetric Michael addition of α-cyanoketones to α,β-unsaturated aldehydes to form chiral dihydropyrans catalyzed by l-diphenylprolinol trimethylsilyl ether is presented. A series of 3,4-dihydropyrans were obtained in excellent yields (up to 91%) and enantioselectivities (up to 98% ee). A plausible mechanism for this process is proposed.
Figure optionsDownload as PowerPoint slide
(4S)-6-(4-Bromophenyl)-2-hydroxy-4-phenyl-3,4-dihydro-2H-pyran-5-carbonitrileC18H14BrNO2[α]D30 = +22.6 (c 1.30, CHCl3)Source of chirality: Asymmetric catalysisAbsolute configuration: (S)
(4S)-6-(4-Bromophenyl)-2-hydroxy-4-(p-tolyl)-3,4-dihydro-2H-pyran-5-carbonitrileC19H16BrNO2[α]D24 = +4.6 (c 0.85, CHCl3)Source of chirality: Asymmetric catalysisAbsolute configuration: (S)
(4S)-6-(4-Bromophenyl)-2-hydroxy-4-(4-methoxyphenyl)-3,4-dihydro-2H-pyran-5-carbonitrileC19H16BrNO3[α]D30 = +13.5 (c 1.25, CHCl3)Source of chirality: Asymmetric catalysisAbsolute configuration: (S)
(4S)-6-(4-Bromophenyl)-2-hydroxy-4-(2-methoxyphenyl)-3,4-dihydro-2H-pyran-5-carbonitrileC19H16NO3Br[α]D30 = −1.0 (c 1.20, CHCl3)Source of chirality: Asymmetric catalysisAbsolute configuration: (S)
(4S)-6-(4-Bromophenyl)-4-(4-chlorophenyl)-2-hydroxy-3,4-dihydro-2H-pyran-5-carbonitrileC18H12BrClNO2[α]D24 = +13.7 (c 1.80, CHCl3)Source of chirality: Asymmetric catalysisAbsolute configuration: (S)
(4S)-4,6-Bis(4-bromophenyl)-2-hydroxy-3,4-dihydro-2H-pyran-5-carbonitrileC18H12Br2NO2[α]D30 = +8.7 (c 1.75, CHCl3)Source of chirality: Asymmetric catalysisAbsolute configuration: (S)
(4R)-6-(4-Bromophenyl)-2-hydroxy-4-methyl-3,4-dihydro-2H-pyran-5-carbonitrileC13H12BrNO2[α]D30 = +37.5 (c 1.00, CHCl3)Source of chirality: Asymmetric catalysisAbsolute configuration: (R)
(4R)-6-(4-Bromophenyl)-4-(furan-2-yl)-2-hydroxy-3,4-dihydro-2H-pyran-5-carbonitrileC16H12BrNO3[α]D24 = +57.1 (c 1.10, CHCl3)Source of chirality: Asymmetric catalysisAbsolute configuration: (R)
(4S)-6-(4-Bromophenyl)-2-hydroxy-4-(4-methoxyphenyl)-3,4-dihydro-2H-pyran-5-carbonitrileC18H15NO2[α]D30 = +31.0 (c 1.05, DMSO)Source of chirality: Asymmetric catalysisAbsolute configuration: (S)
(4S)-6-(2-Bromophenyl)-2-hydroxy-4-phenyl-3,4-dihydro-2H-pyran-5-carbonitrileC18H14NO2Br[α]D30 = +59.0 (c 1.10, MeOH)Source of chirality: Asymmetric catalysisAbsolute configuration: (S)
(4S)-6-(3-Bromophenyl)-2-hydroxy-4-phenyl-3,4-dihydro-2H-pyran-5-carbonitrileC18H14NO2Br[α]D24 = +31.3 (c 1.60, CHCl3)Source of chirality: Asymmetric catalysisAbsolute configuration: (S)
(4S)-2-Hydroxy-4-phenyl-6-(p-tolyl)-3,4-dihydro-2H-pyran-5-carbonitrileC19H17NO2[α]D30 = +22.6 (c 0.75, CHCl3)Source of chirality: Asymmetric catalysisAbsolute configuration: (S)
(4S)-2-Hydroxy-6-(4-methoxyphenyl)-4-phenyl-3,4-dihydro-2H-pyran-5-carbonitrileC19H17NO3[α]D30 = −20.0 (c 1.20, MeOH)Source of chirality: Asymmetric catalysisAbsolute configuration: (S)
(4S)-6-(4-Fluorophenyl)-2-hydroxy-4-phenyl-3,4-dihydro-2H-pyran-5-carbonitrileC18H14FNO2[α]D24 = +8.1 (c 1.05, MeOH)Source of chirality: Asymmetric catalysisAbsolute configuration: (S)
Journal: Tetrahedron: Asymmetry - Volume 25, Issues 10–11, 31 May 2014, Pages 796–801