| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345250 | 1500347 | 2014 | 4 صفحه PDF | دانلود رایگان |
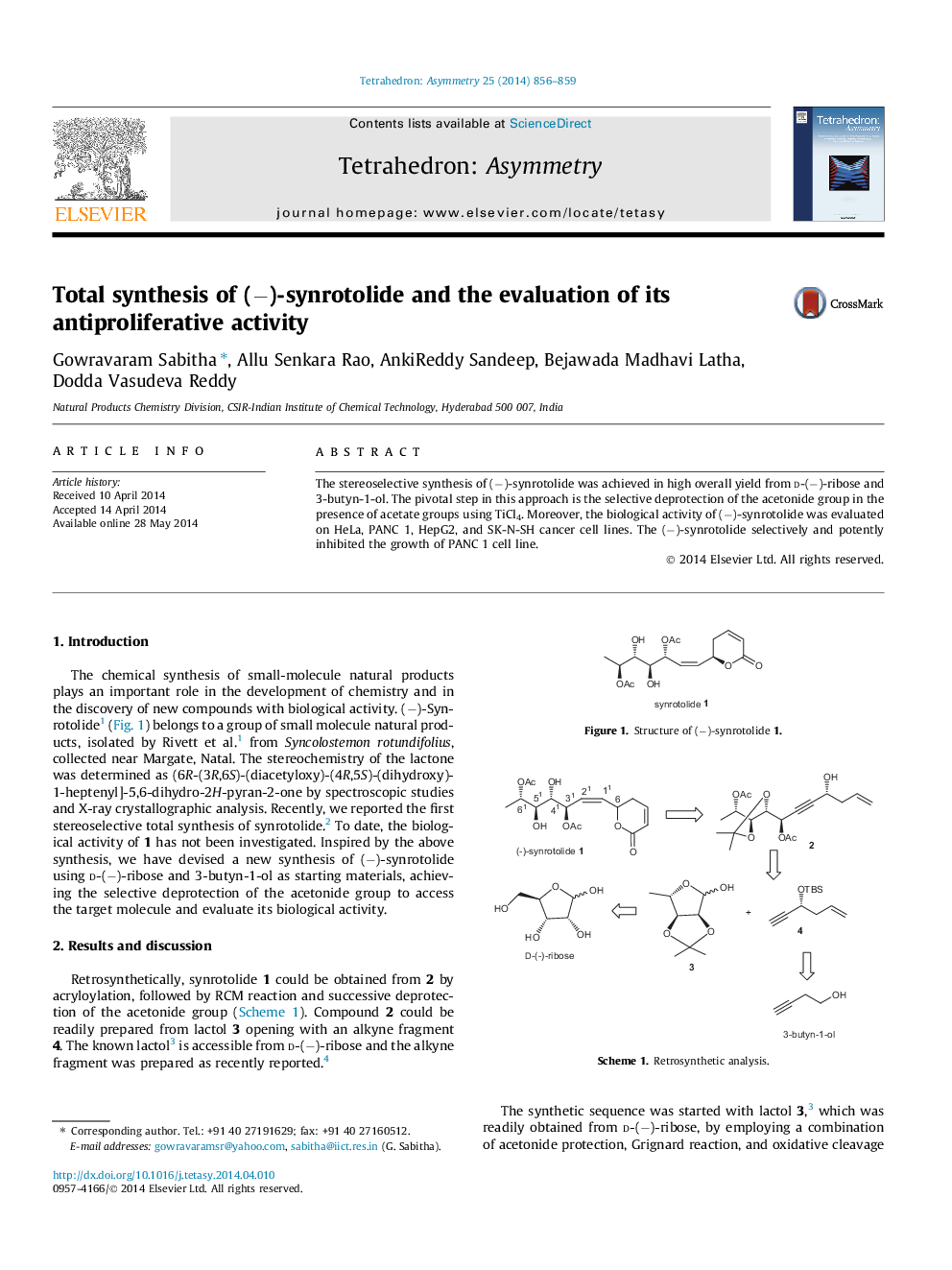
The stereoselective synthesis of (−)-synrotolide was achieved in high overall yield from d-(−)-ribose and 3-butyn-1-ol. The pivotal step in this approach is the selective deprotection of the acetonide group in the presence of acetate groups using TiCl4. Moreover, the biological activity of (−)-synrotolide was evaluated on HeLa, PANC 1, HepG2, and SK-N-SH cancer cell lines. The (−)-synrotolide selectively and potently inhibited the growth of PANC 1 cell line.
Figure optionsDownload as PowerPoint slide
(1R,4R)-4-(tert-Butyldimethylsilyloxy)-1-((4R,5S)-5-((S)-1-hydroxyethyl)-2,2-dimethyl-1,3-dioxolan-4-yl)hept-6-en-2-yn-1-olC20H36O5Si[α]D25 = +51.6 (c 1.2, CHCl3)Absolute configuration: (1R,4R,4R,5S,S)Source of chirality: d-ribose
(S)-1-((4S,5R)-5-((1R,4R)-1-Acetoxy-4-(tert-butyldimethylsilyloxy)hept-6-en-2-ynyl)-2,2-dimethyl-1,3-dioxolan-4-yl)ethyl acetateC24H40O7Si[α]D25 = +15.2 (c 1.0, CHCl3)Absolute configuration: (S,4S,5R,1R,4R)Source of chirality: d-ribose
(S)-1-((4S,5R)-5-((1R,4R)-1-Acetoxy-4-hydroxyhept-6-en-2-ynyl)-2,2-dimethyl-1,3-dioxolan-4-yl)ethyl acetateC18H26O7[α]D25 = +12.6 (c 0.8, CHCl3)Absolute configuration: (S,4S,5R,1R,4R)Source of chirality: d-ribose
(4R,7R)-7-Acetoxy-7-((4R,5S)-5-((S)-1-acetoxyethyl)-2,2-dimethyl-1,3-dioxolan-4-yl)hept-1-en-5-yn-4-yl acrylateC21H28O8[α]D25 = +61.3 (c 0.65, CHCl3)Absolute configuration: (4R,7R,4R,5S,S)Source of chirality: d-ribose
(S)-1-((4S,5R)-5-((R)-1-Acetoxy-3-((R)-6-oxo-3,6-dihydro-2H-pyran-2-yl)prop-2-ynyl)-2,2-dimethyl-1,3-dioxolan-4-yl)ethylC19H24O8[α]D25 = +16.5 (c 1.5, CHCl3)Absolute configuration: (S,4S,5R,R,R)Source of chirality: d-ribose
(S)-1-((4S,5R)-5-((R,Z)-1-Acetoxy-3-((R)-6-oxo-3,6-dihydro-2H-pyran-2-yl)allyl)-2,2-dimethyl-1,3-dioxolan-4-yl)ethyl acetateC16H20O8[α]D25 = −7.3 (c 0.35, CHCl3)Absolute configuration: (S,4S,5R,R,Z,R)Source of chirality: d-ribose
(2S,3R,4S,5R,Z)-3,4-Aihydroxy-7-((R)-6-oxo-3,6-dihydro-2H-pyran-2-yl)hept-6-ene-2,5-diyl diacetateC16H22O8[α]D25 = −25.8 (c 0.20, CHCl3)Absolute configuration: (2S,3R,4S,5R,Z,R)Source of chirality: d-ribose
Journal: Tetrahedron: Asymmetry - Volume 25, Issues 10–11, 31 May 2014, Pages 856–859