| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345337 | 980191 | 2015 | 5 صفحه PDF | دانلود رایگان |
عنوان انگلیسی مقاله ISI
Catalytic asymmetric synthesis of (S,4E,15Z)-docosa-4,15-dien-1-yn-3-ol, an antitumor marine natural product
دانلود مقاله + سفارش ترجمه
دانلود مقاله ISI انگلیسی
رایگان برای ایرانیان
موضوعات مرتبط
مهندسی و علوم پایه
شیمی
شیمی معدنی
پیش نمایش صفحه اول مقاله
چکیده انگلیسی
An efficient enantioselective total synthesis of an antitumor marine natural product (S,4E,15Z)-docosa-4,15-dien-1-yn-3-ol 1 with 96% ee and 15% overall yield has been achieved; this is the first preparation of 1 via asymmetric catalytic strategy. The key steps involve the asymmetric addition of trimethylsilylacetylene to a diolefinc aldehyde using a (R,R)-ProPhenol ligand and a zipper reaction of an alkyne.
Figure optionsDownload as PowerPoint slide
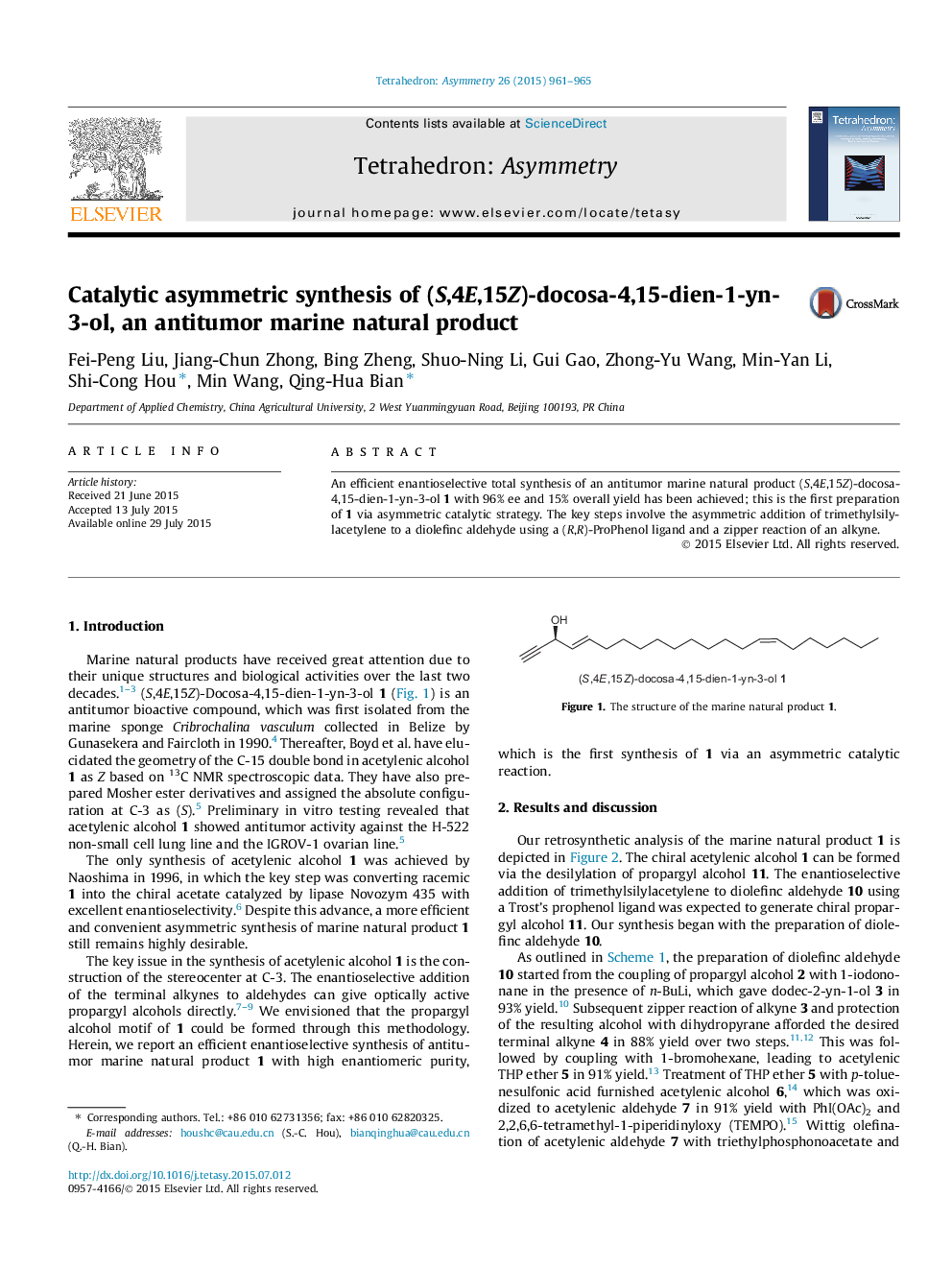
(S,4E,15Z)-Docosa-4,15-dien-1-yn-3-olC22H38OEe = 96%[α]D20 = +18.4 (c 0.10, MeOH)Source of chirality: (R,R)-ProPhenolAbsolute configuration: (S)
ناشر
Database: Elsevier - ScienceDirect (ساینس دایرکت)
Journal: Tetrahedron: Asymmetry - Volume 26, Issue 17, 15 September 2015, Pages 961–965
Journal: Tetrahedron: Asymmetry - Volume 26, Issue 17, 15 September 2015, Pages 961–965
نویسندگان
Fei-Peng Liu, Jiang-Chun Zhong, Bing Zheng, Shuo-Ning Li, Gui Gao, Zhong-Yu Wang, Min-Yan Li, Shi-Cong Hou, Min Wang, Qing-Hua Bian,