| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345491 | 980204 | 2007 | 8 صفحه PDF | دانلود رایگان |
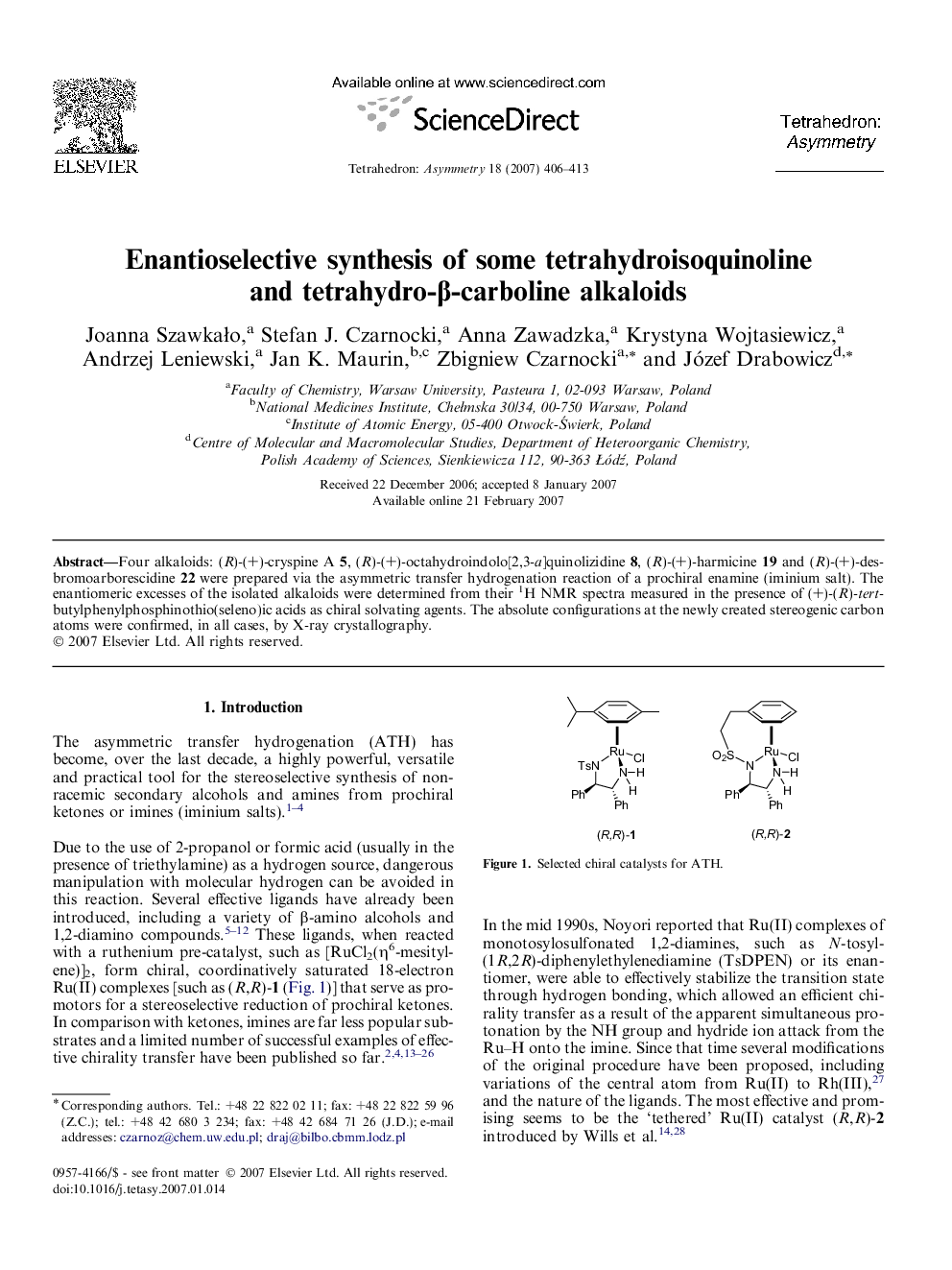
Four alkaloids: (R)-(+)-cryspine A 5, (R)-(+)-octahydroindolo[2,3-a]quinolizidine 8, (R)-(+)-harmicine 19 and (R)-(+)-desbromoarborescidine 22 were prepared via the asymmetric transfer hydrogenation reaction of a prochiral enamine (iminium salt). The enantiomeric excesses of the isolated alkaloids were determined from their 1H NMR spectra measured in the presence of (+)-(R)-tert-butylphenylphosphinothio(seleno)ic acids as chiral solvating agents. The absolute configurations at the newly created stereogenic carbon atoms were confirmed, in all cases, by X-ray crystallography.
Figure optionsDownload as PowerPoint slide
9,10-Dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinoline N-oxideC15H21NO3[α]D23=-14.3 (c 1, CHCl3)Source of chirality: asymmetric transfer hydrogenationAbsolute configuration: (R)
1,2,3,4,6,7,12,12b-Octahydro-indolo[2,3-a]quinolizineC15H18N2[α]D23=+82.7 (c 1, CH3OH) (98.5% ee)Source of chirality: asymmetric transfer hydrogenationAbsolute configuration: (R)
Journal: Tetrahedron: Asymmetry - Volume 18, Issue 3, 28 February 2007, Pages 406–413