| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345556 | 980209 | 2007 | 7 صفحه PDF | دانلود رایگان |
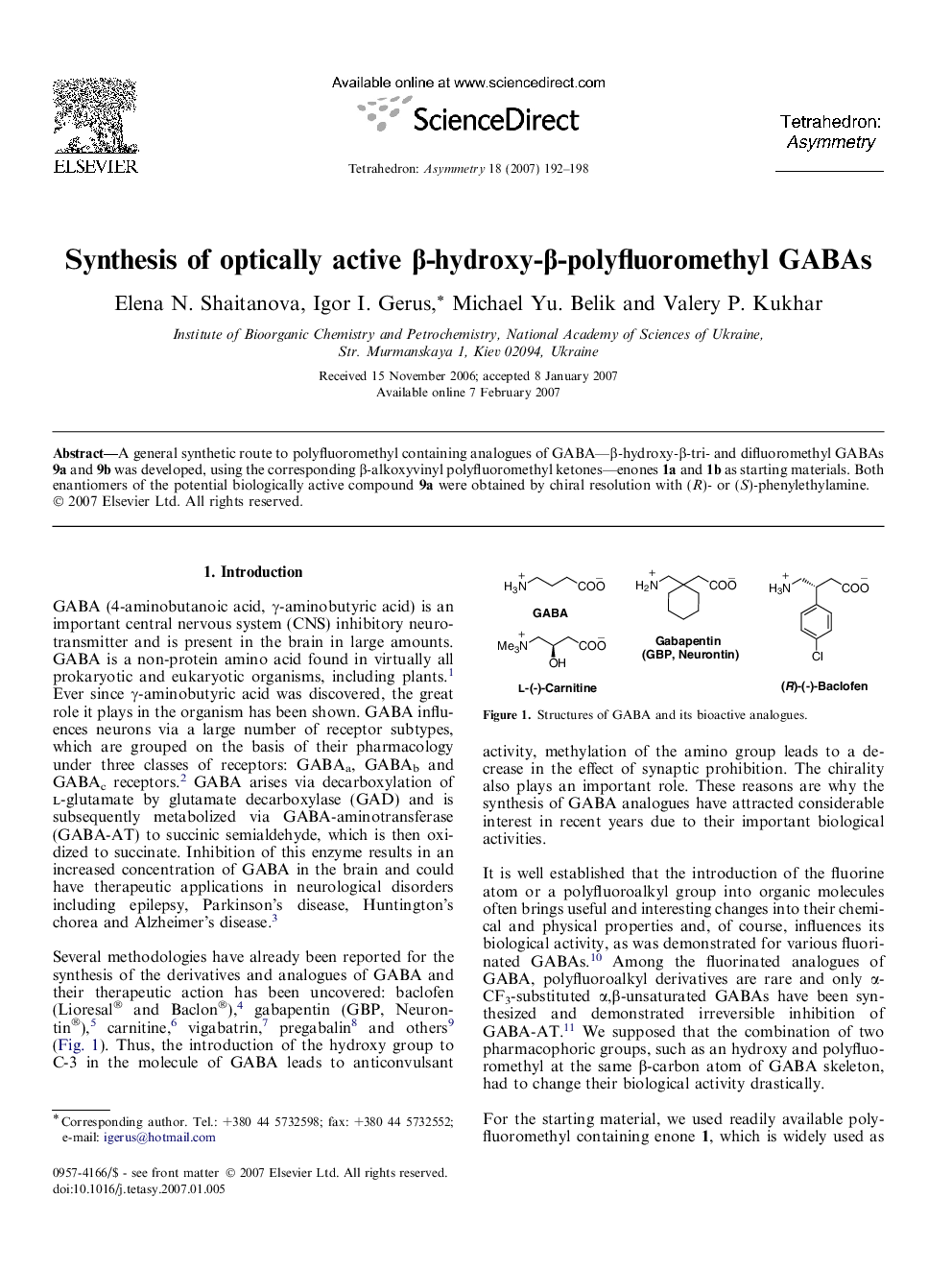
A general synthetic route to polyfluoromethyl containing analogues of GABA—β-hydroxy-β-tri- and difluoromethyl GABAs 9a and 9b was developed, using the corresponding β-alkoxyvinyl polyfluoromethyl ketones—enones 1a and 1b as starting materials. Both enantiomers of the potential biologically active compound 9a were obtained by chiral resolution with (R)- or (S)-phenylethylamine.
A general synthetic route to racemic and optically active β-polyfluoromethyl-β-hydroxy-containing analogues of GABA was developed, using β-alkoxyvinyl polyfluoromethyl ketones as starting materials.Figure optionsDownload as PowerPoint slide
(3R)-4,4,4-Trifluoro-3-hydroxy-3-(aminomethyl)-butanoic acidC6H10F3NO3Ee >99.9%[α]D25=+32.2 (c 0.8, 1% NH4OH in H2O)Source of chirality: chiral resolutionAbsolute configuration: (3R)
Journal: Tetrahedron: Asymmetry - Volume 18, Issue 2, 14 February 2007, Pages 192–198