| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345577 | 980210 | 2013 | 7 صفحه PDF | دانلود رایگان |
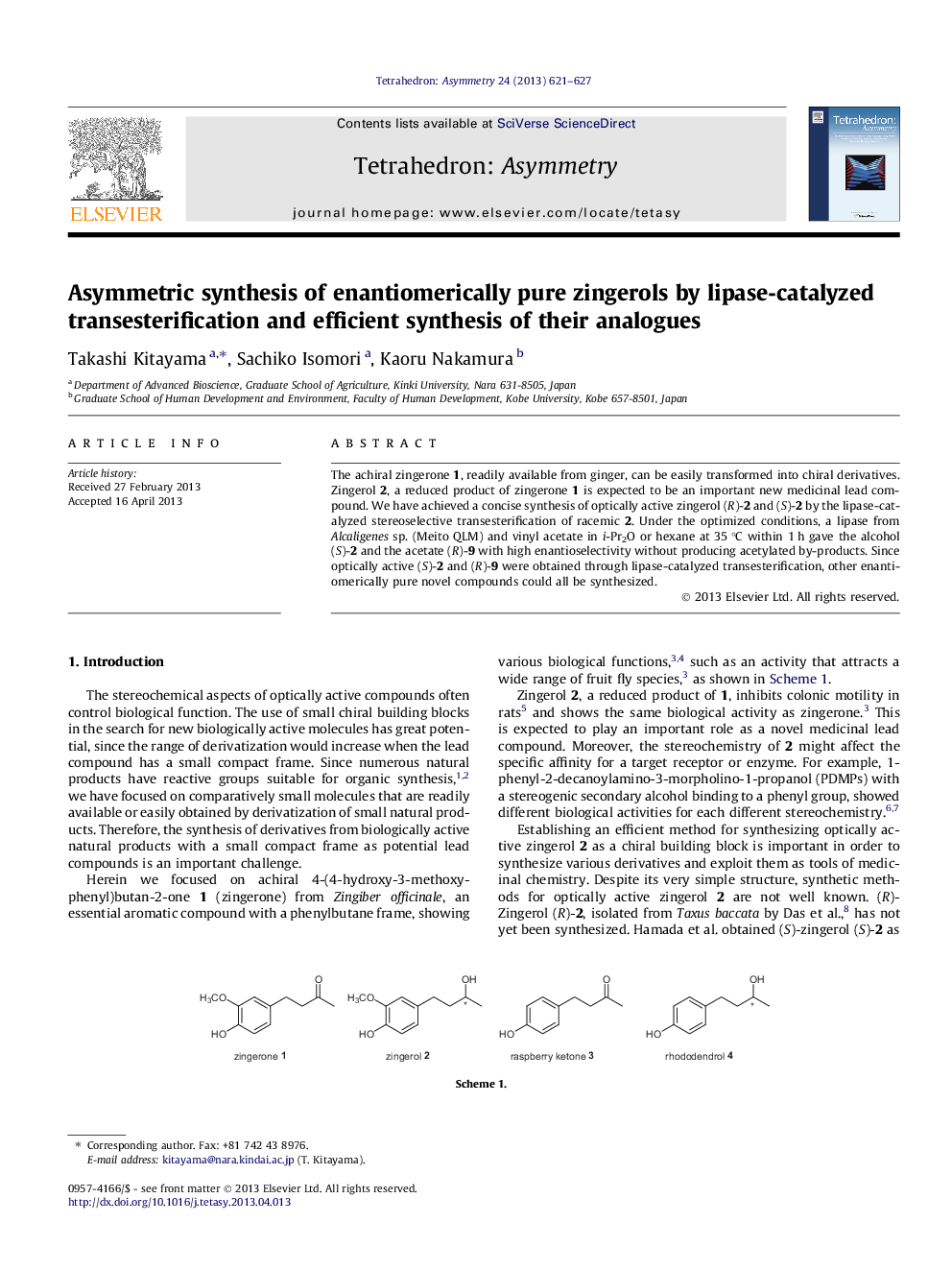
The achiral zingerone 1, readily available from ginger, can be easily transformed into chiral derivatives. Zingerol 2, a reduced product of zingerone 1 is expected to be an important new medicinal lead compound. We have achieved a concise synthesis of optically active zingerol (R)-2 and (S)-2 by the lipase-catalyzed stereoselective transesterification of racemic 2. Under the optimized conditions, a lipase from Alcaligenes sp. (Meito QLM) and vinyl acetate in i-Pr2O or hexane at 35 °C within 1 h gave the alcohol (S)-2 and the acetate (R)-9 with high enantioselectivity without producing acetylated by-products. Since optically active (S)-2 and (R)-9 were obtained through lipase-catalyzed transesterification, other enantiomerically pure novel compounds could all be synthesized.
Figure optionsDownload as PowerPoint slide
(S)-4-(4′-hydroxy-3′-methoxyphenyl)-2-butanolC11H16O3Ee >99%[α]D24=+14.1 (c 0.91, EtOH)Source of chirality: lipase resolutionAbsolute configuration: (S)
(R)-4-(4′-hydroxy-3′-methoxyphenyl)-2-butanolC11H16O3Ee >99%[α]D24=-15.1 (c 1.10, EtOH)Source of chirality: lipase resolutionAbsolute configuration: (R)
(S)-4-(4′-Acetyloxy-3′-methoxyphenyl)-2-butanolC13H18O4Ee >99%[α]D24 = +12.9 (c 0.97, EtOH)Source of chirality: lipase resolutionAbsolute configuration: (S)
(R)-4-(4′-Acetyloxy-3′-methoxyphenyl)-2-butanolC13H18O4Ee >99%[α]D24=-13.9 (c 0.84, EtOH)Source of chirality: lipase resolutionAbsolute configuration: (R)
(S)-4-(4′-Hydroxy-3′-methoxyphenyl)-2-butyl acetateC13H18O4Ee >99%[α]D24=-1.3 (c 1.14, EtOH)Source of chirality: lipase resolutionAbsolute configuration: (S)
(R)-4-(4′-Hydroxy-3′-methoxyphenyl)-2-butyl acetateC13H18O4Ee >99%[α]D24=+1.7 (c 1.00, EtOH)Source of chirality: lipase resolutionAbsolute configuration: (R)
(S)-4-(4′-Acetyloxy-3′-methoxyphenyl)-2-butyl acetateC15H20O5Ee >99%[α]D24=-3.9 (c 0.96, EtOH)Source of chirality: lipase resolutionAbsolute configuration: (S)
(R)-4-(4′-Acetyloxy-3′-methoxyphenyl)-2-butyl acetateC15H20O5Ee >99%[α]D24=+4.8 (c 1.00, EtOH)Source of chirality: lipase resolutionAbsolute configuration: (R)
Journal: Tetrahedron: Asymmetry - Volume 24, Issue 11, 15 June 2013, Pages 621–627