| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345601 | 1500328 | 2016 | 5 صفحه PDF | دانلود رایگان |
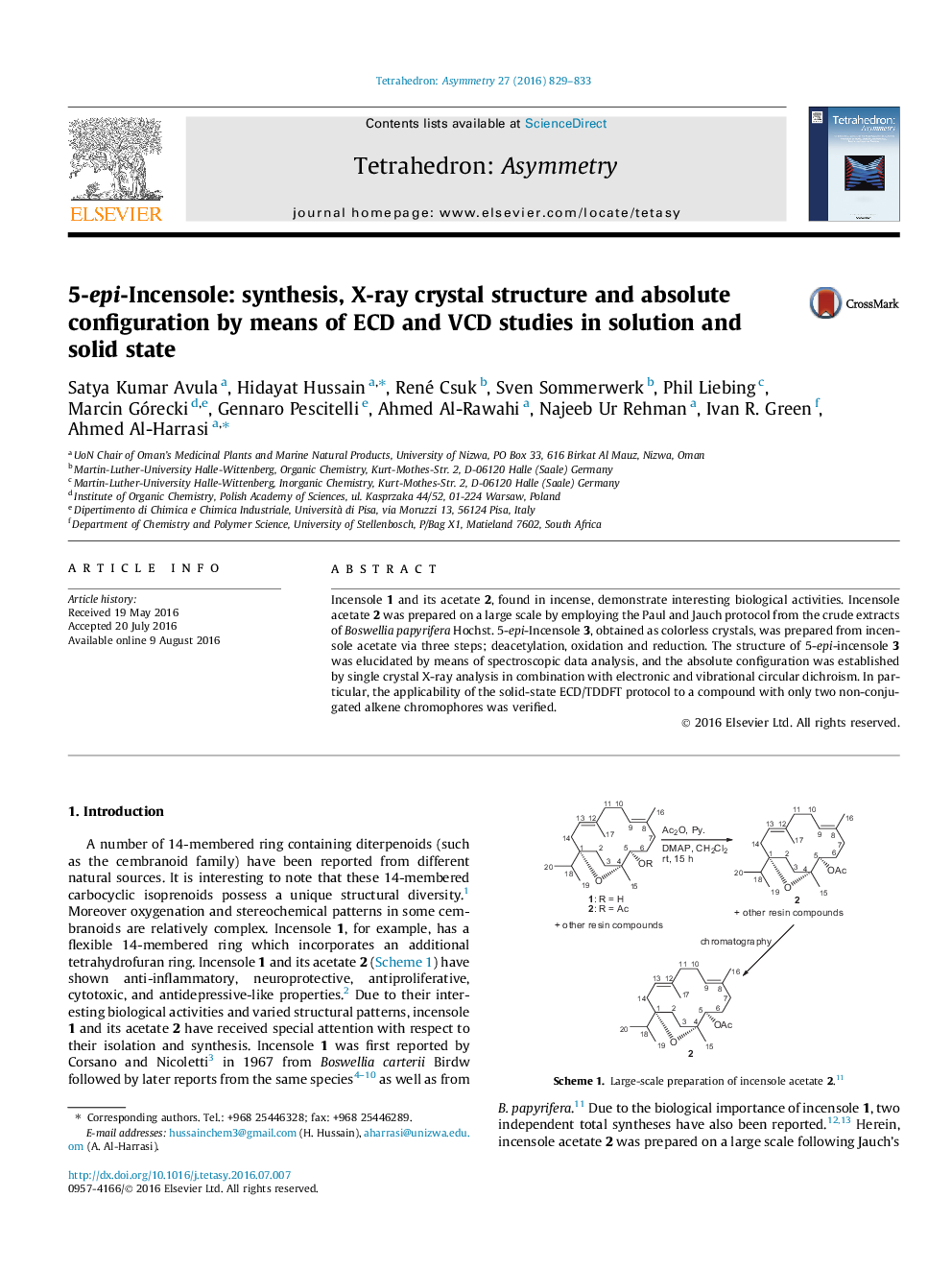
Incensole 1 and its acetate 2, found in incense, demonstrate interesting biological activities. Incensole acetate 2 was prepared on a large scale by employing the Paul and Jauch protocol from the crude extracts of Boswellia papyrifera Hochst. 5-epi-Incensole 3, obtained as colorless crystals, was prepared from incensole acetate via three steps; deacetylation, oxidation and reduction. The structure of 5-epi-incensole 3 was elucidated by means of spectroscopic data analysis, and the absolute configuration was established by single crystal X-ray analysis in combination with electronic and vibrational circular dichroism. In particular, the applicability of the solid-state ECD/TDDFT protocol to a compound with only two non-conjugated alkene chromophores was verified.
Figure optionsDownload as PowerPoint slide
(+)-(1S,4R,5R)-5-epi-IncensoleC20H34O2Absolute configuration: (1S,4R,5R) (assigned by NMR, X-ray analysis, ECD, VCD, TDDFT)[α]D25 = −11.8 (c 0.06, CH2Cl2)
Journal: Tetrahedron: Asymmetry - Volume 27, Issues 17–18, 1 October 2016, Pages 829–833