| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345634 | 980214 | 2006 | 9 صفحه PDF | دانلود رایگان |
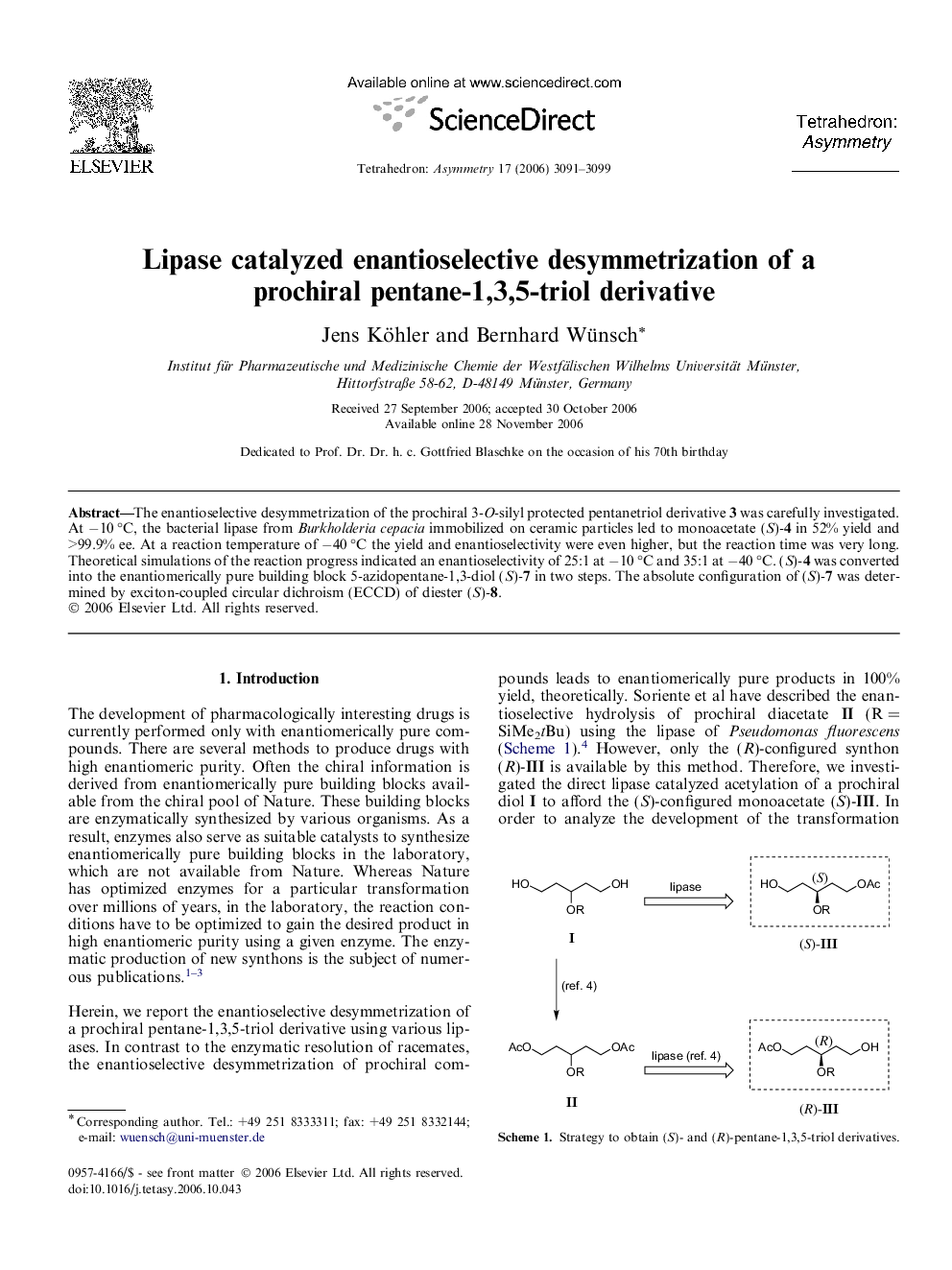
The enantioselective desymmetrization of the prochiral 3-O-silyl protected pentanetriol derivative 3 was carefully investigated. At −10 °C, the bacterial lipase from Burkholderia cepacia immobilized on ceramic particles led to monoacetate (S)-4 in 52% yield and >99.9% ee. At a reaction temperature of −40 °C the yield and enantioselectivity were even higher, but the reaction time was very long. Theoretical simulations of the reaction progress indicated an enantioselectivity of 25:1 at −10 °C and 35:1 at −40 °C. (S)-4 was converted into the enantiomerically pure building block 5-azidopentane-1,3-diol (S)-7 in two steps. The absolute configuration of (S)-7 was determined by exciton-coupled circular dichroism (ECCD) of diester (S)-8.
Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron: Asymmetry - Volume 17, Issue 22, 27 November 2006, Pages 3091–3099