| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345660 | 980215 | 2013 | 6 صفحه PDF | دانلود رایگان |
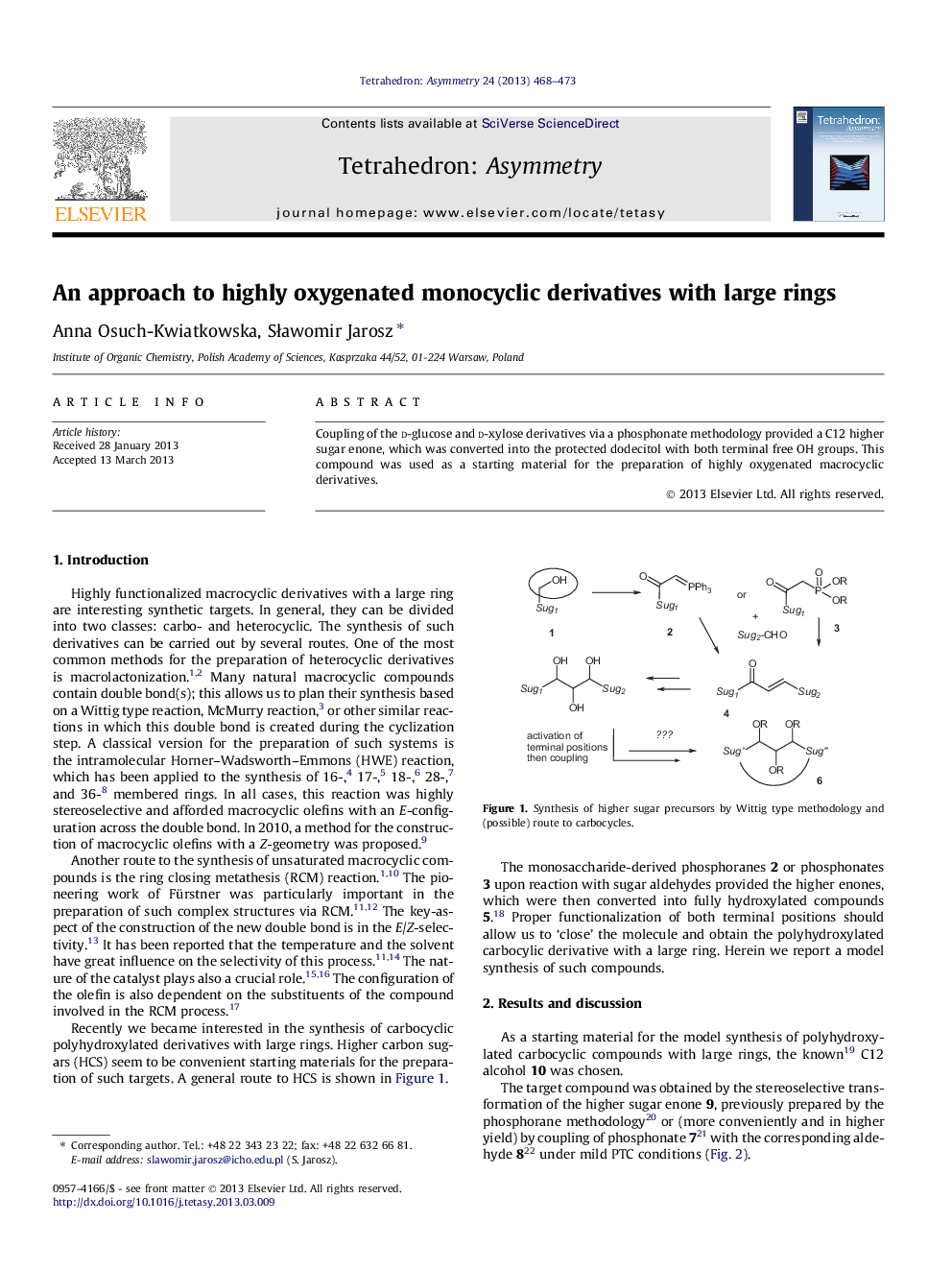
Coupling of the d-glucose and d-xylose derivatives via a phosphonate methodology provided a C12 higher sugar enone, which was converted into the protected dodecitol with both terminal free OH groups. This compound was used as a starting material for the preparation of highly oxygenated macrocyclic derivatives.
Figure optionsDownload as PowerPoint slide
Methyl 2,3,4,6,7,8,9,10,11-nona-O-benzyl-l-threo-l-manno-α-d-gluco-dodeca-1,5-pyranosideC76H80O12[α]Drt=+14.0 (c 0.5, CHCl3)Source of chirality: d-glucose and d-gluconic acidAbsolute configuration: (1S,2R,3S,4S,5R,6R,7R,8S,9S,10R,11S)
2,3,4,5,6,7,8,9,10,11-Deca-O-benzyl-l-threo-l-manno-d-gluco-dodecitolC82H86O12[α]Drt=+2.0 (c 0.5, CHCl3)Source of chirality: d-glucose and d-gluconic acidAbsolute configuration: (2S,3R,4S,5S,6S,7R,8S,9S,10R,11S)
(3S,4R,5S,6S,7S,8R,9S,10S,11R,12S)-3,4,5,6,7,8,9,10,11,12-Deca(benzyloxy)-tetradeca-1,13-dieneC84H86O10[α]Drt=+23.0 (c 0.5, CHCl3)Source of chirality: d-glucose and d-gluconic acidAbsolute configuration: (3S,4R,5S,6S,7S,8R,9S,10S,11R,12S)
1-Deoxy-2,4,6,7,8,9,10,11-octa-O-benzyl-l-threo-l-manno-α-d-allo-dodeca-1,5-pyranosideC68H72O11[α]Drt=-28.0 (c 1.3, CHCl3)Source of chirality: d-glucose and d-gluconic acidAbsolute configuration: (2S,3S,4S,5R,6R,7R,8S,9S,10R,11S)
(2R,3S,4R,5R,6R,7S,8R,9R,10S,11R)-2,3,4,5,6,7,8,9,10,11-Deca(benzyloxy)-dodecanedialC82H82O12[α]Drt=+20.0 (c 1.0, CHCl3)Source of chirality: d-glucose and d-gluconic acidAbsolute configuration: (2R,3S,4R,5R,6R,7S,8R,9R,10S,11R)
(6S,7R,8S,9S,10S,11R,12S,13S,14R,15S)-6,7,8,9,10,11,12,13,14,15-Deca(benzyloxy)-4,17-dioxan-icosa-1,19-dieneC88H94O12[α]Drt=-5.0 (c 0.25, CHCl3)Source of chirality: d-glucose and d-gluconic acidAbsolute configuration: (6S,7R,8S,9S,10S,11R,12S,13S,14R,15S)
(8S,9R,10S,11S,12S,13R,14S,15S,16R,17S)-8,9,10,11,12,13,14,15,16,17-Deca(benzyloxy)-1,6-dioxan-cyclooctadecaC86H92O12[α]Drt=+7.0 (c 0.6, CHCl3)Source of chirality: d-glucose and d-gluconic acidAbsolute configuration: (8S,9R,10S,11S,12S,13R,14S,15S,16R,17S)
Journal: Tetrahedron: Asymmetry - Volume 24, Issue 8, 30 April 2013, Pages 468–473