| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345761 | 980219 | 2014 | 5 صفحه PDF | دانلود رایگان |
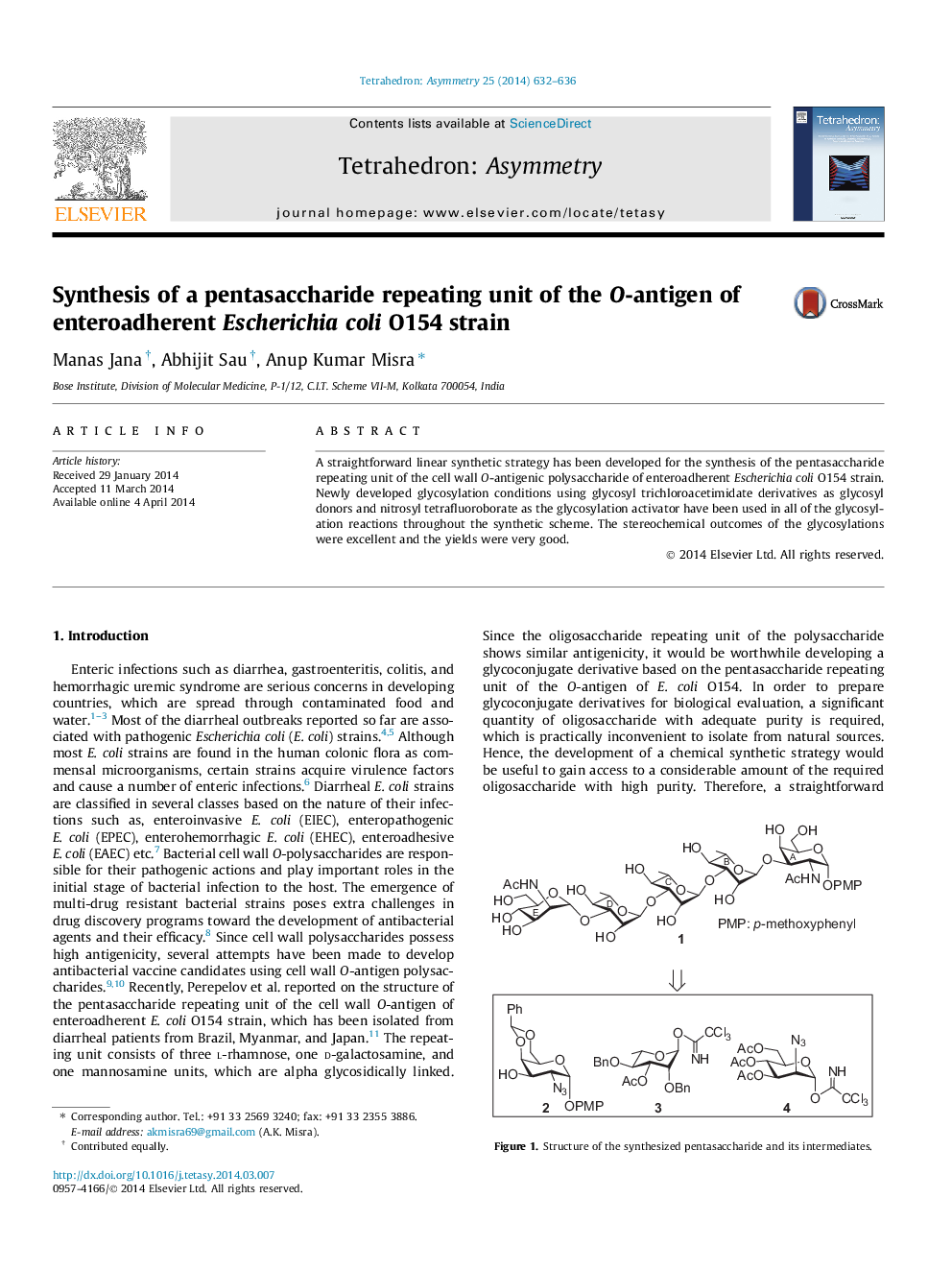
A straightforward linear synthetic strategy has been developed for the synthesis of the pentasaccharide repeating unit of the cell wall O-antigenic polysaccharide of enteroadherent Escherichia coli O154 strain. Newly developed glycosylation conditions using glycosyl trichloroacetimidate derivatives as glycosyl donors and nitrosyl tetrafluoroborate as the glycosylation activator have been used in all of the glycosylation reactions throughout the synthetic scheme. The stereochemical outcomes of the glycosylations were excellent and the yields were very good.
Figure optionsDownload as PowerPoint slide
p-Methoxyphenyl (3-O-acetyl-2,4-di-O-benzyl-α-l-rhamnopyranosyl)-(1→3)-2-azido-4,6-O-benzylidene-2-deoxy-α-d-galactopyranosideC42H45N3O11[α]D25=+49 (c 1.0, CHCl3).Source of chirality: l-rhamnose, d-galactosamine.
p-Methoxyphenyl (2,4-di-O-benzyl-α-l-rhamnopyranosyl)-(1→3)-2-azido-4,6-O-benzylidene-2-deoxy-α-d-galactopyranosideC40H43N3O10[α]D25=+64 (c 1.0, CHCl3).Source of chirality: l-rhamnose, d-galactosamine.
p-Methoxyphenyl (3-O-acetyl-2,4-di-O-benzyl-α-l-rhamnopyranosyl)-(1→3)-(2,4-di-O-benzyl-α-l-rhamnopyranosyl)-(1→3)-2-azido-4,6-O-benzylidene-2-deoxy-α-d-galactopyranosideC62H67N3O15[α]D25=+54 (c 1.0, CHCl3).Source of chirality: l-rhamnose, d-galactosamine.
p-Methoxyphenyl (2,4-di-O-benzyl-α-l-rhamnopyranosyl)-(1→3)-(2,4-di-O-benzyl-α--rhamnopyranosyl)-(1→3)-2-azido-4,6-O-benzylidene-2-deoxy-α-d-galactopyranosideC60H65N3O14[α]D25=+55 (c 1.0, CHCl3).Source of chirality: l-rhamnose, d-galactosamine.
p-Methoxyphenyl (3-O-acetyl-2,4-di-O-benzyl-α-l-rhamnopyranosyl)-(1→3)-(2,4-di-O-benzyl-α-l-rhamnopyranosyl)-(1→3)-(2,4-di-O-benzyl-α-l-rhamnopyranosyl)-(1→3)-2-azido-4,6-O-benzylidene-2-deoxy-α-d-galactopyranosideC82H89N3O19[α]D25=+71 (c 1.0, CHCl3).Source of chirality: l-rhamnose, d-galactosamine.
p-Methoxyphenyl (2,4-di-O-benzyl-α-l-rhamnopyranosyl)-(1→3)-(2,4-di-O-benzyl-α-l-rhamnopyranosyl)-(1→3)-(2,4-di-O-benzyl-α-l-rhamnopyranosyl)-(1→3)-2-azido-4,6-O-benzylidene-2-deoxy-α-d-galactopyranosideC80H87N3O18[α]D25=+115 (c 1.0, CHCl3).Source of chirality: l-rhamnose, d-galactosamine.
p-Methoxyphenyl (3,4,6-tri-O-acetyl-2-azido-2-deoxy-α-D-mannopyranosyl)-(1→3)-(2,4-di-O-benzyl-α-L-rhamnopyranosyl)-(1→3)-(2,4-di-O-benzyl-α-L-rhamnopyranosyl)-(1→3)-(2,4-di-O-benzyl-α-L-rhamnopyranosyl)-(1→3)-2-azido-4,6-O-benzylidene-2-deoxy-α-D-galactopyranoside (11)C92H102N6O25[α]D25=+44 (c 1.0, CHCl3).Source of chirality: D-glucose, L-rhamnose, D-galactosamine.
p-Methoxyphenyl (2-acetamido-2-deoxy-α-D-mannopyranosyl)-(1→3)-(α-L-rhamnopyranosyl)-(1→3)-(α-L-rhamnopyranosyl)-(1→3)-(α-L-rhamnopyranosyl)-(1→3)-2-acetamido-2-deoxy-α-D-galactopyranoside (1)C41H64N2O24[α]D25=-130 (c 1.0, H2O).Source of chirality: D-glucose, L-rhamnose, D-galactosamine.
Journal: Tetrahedron: Asymmetry - Volume 25, Issue 8, 30 April 2014, Pages 632–636