| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345778 | 980220 | 2013 | 6 صفحه PDF | دانلود رایگان |
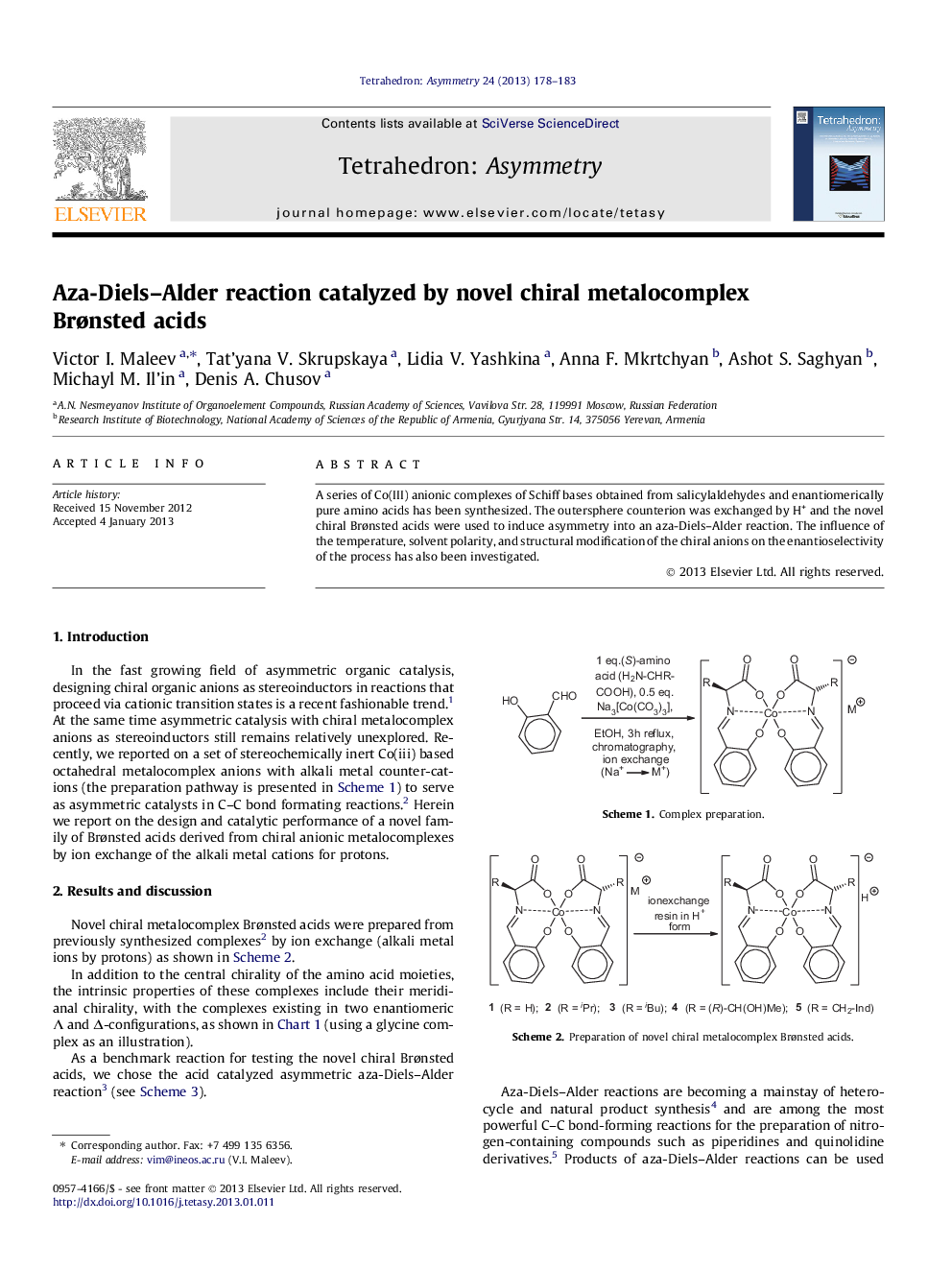
A series of Co(III) anionic complexes of Schiff bases obtained from salicylaldehydes and enantiomerically pure amino acids has been synthesized. The outersphere counterion was exchanged by H+ and the novel chiral Brønsted acids were used to induce asymmetry into an aza-Diels–Alder reaction. The influence of the temperature, solvent polarity, and structural modification of the chiral anions on the enantioselectivity of the process has also been investigated.
Figure optionsDownload as PowerPoint slide
Hydrogen Δ-[bis(N-salicylidene-(S)-leucinato)cobaltate(iii)]Ee >99%[α]D25=-5692 (c 0.05, MeOH)Source of chirality: synthesis from (S)-leucineAbsolute configuration: (Δ,S,S)
(1S,3R,4S)-2,3-Diphenyl-2-azabicyclo[2.2.2]octan-5-oneEe 98%[α]D25=+74.6 (c 0.1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (1S,3R,4S)
(1S,3R,4S)-3-(4-Methoxyphenyl)-2-phenyl-2-azabicyclo[2.2.2]octan-5-oneEe 94%[α]D25=+36.4 (c 0.11, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (1S,3R,4S)
(1S,3R,4S)-3-(3-Chlorophenyl)-2-phenyl-2-azabicyclo[2.2.2]octan-5-oneEe 71%[α]D25=+50.8 (c 0.33, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (1S,3R,4S)
(1S,3R,4S)-2-(4-Chlorophenyl)-3-phenyl-2-azabicyclo[2.2.2]octan-5-oneEe 76%[α]D25=+53.6 (c 0.1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (1S,3R,4S)
Journal: Tetrahedron: Asymmetry - Volume 24, Issue 4, 28 February 2013, Pages 178–183