| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345871 | 1500338 | 2015 | 5 صفحه PDF | دانلود رایگان |
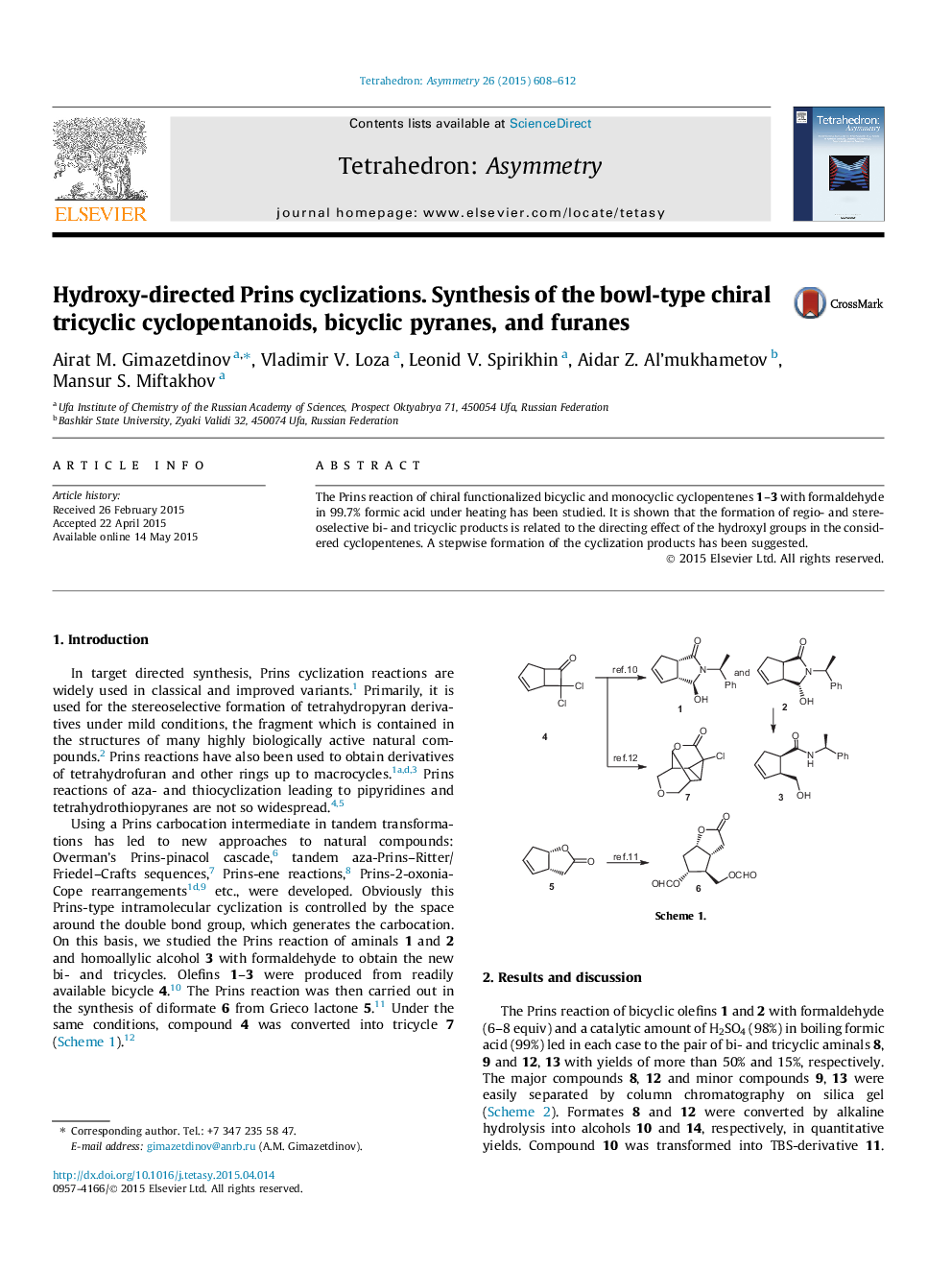
The Prins reaction of chiral functionalized bicyclic and monocyclic cyclopentenes 1–3 with formaldehyde in 99.7% formic acid under heating has been studied. It is shown that the formation of regio- and stereoselective bi- and tricyclic products is related to the directing effect of the hydroxyl groups in the considered cyclopentenes. A stepwise formation of the cyclization products has been suggested.
Figure optionsDownload as PowerPoint slide
(−)-(2aS,3R,4aR,6bS)-5-Oxo-6-[(1S)-1-phenylethyl]octahydro-2H-1-oxa-6-azacyclopenta-[cd]pentalen-3-yl-formateC17H19NO4[α]D20 = −129.0 (c 1.0, CH2Cl2)Source of chirality: (−)-(S)-phenylethylamineAbsolute configuration: (2aS,3R,4aR,6bS)
(−)-(2aS,4aR,6bS)-6-[(1S)-1-Phenylethyl]-2,2a,4a,6,6a,6b-hexahydro-5H-1-oxa-6-aza-cyclopenta[cd]pentalen-5-oneC16H17NO2[α]D20 = −11.7 (c 1.5, CH2Cl2)Source of chirality: (−)-(S)-phenylethylamineAbsolute configuration: (2aS,4aR,6bS)
(−)-(2aR,3S,4aS,6bR)-5-Oxo-6-[(1S)-1-phenylethyl]octahydro-2H-1-oxa-6-azacyclopenta-[cd]pentalen-3-yl-formateC17H19NO4[α]D20 = −61.1 (c 1.15, CH2Cl2)Source of chirality: (−)-(S)-phenylethylamineAbsolute configuration: (2aR,3S,4aS,6bR)
(−)-(2aR,4aS,6bR)-6-[(1S)-1-Phenylethyl]-2,2a,4a,6,6a,6b-hexahydro-5H-1-oxa-6-aza-cyclopenta[cd]pentalen-5-oneC16H17NO2[α]D20 = −63 (c 1.05, CH2Cl2)Source of chirality: (−)-(S)-phenylethylamineAbsolute configuration: (2aR,4aS,6bR)
(−)-(2aS,3R,4aR,6bS)-3-Hydroxy-6-[(1S)-1-phenylethyl]octa-hydro-5H-1-oxa-6-azacyclo-penta[cd]pentalen-5-oneC16H19NO3[α]D20 = −64.5 (c 0.8, CH3OH)Source of chirality: (−)-(S)-phenylethylamineAbsolute configuration: (2aS,3R,4aR,6bS)
(−)-(2aR,3S,4aS,6bR)-3-Hydroxy-6-[(1S)-1-phenylethyl]octa-hydro-5H-1-oxa-6-azacyclo-penta[cd]pentalen-5-oneC16H19NO3[α]D20 = −55.0 (c 1.075, CH3OH)Source of chirality: (−)-(S)-phenylethylamineAbsolute configuration: (2aR,3S,4aS,6bR)
(−)-(2aS,3R,4aR,6bS)-3-(tert-{Butyl(dimethyl)silyl}oxy)-6-[(1S)-1-phenylethyl]octahydro-5H-1-oxa-6-azacyclopenta[cd]pentalen-5-oneC22H33NO3Si[α]D20 = −11.8 (c 1.07, CH2Cl2)Source of chirality: (−)-(S)-phenylethylamineAbsolute configuration: (2aS,3R,4aR,6bS)
(−)-(1R,5S,6R,8R)-8-Hydroxy-N-[(1S)-1-phenylethyl]-3-oxabicyc-lo[3.2.1]octane-6-carboxamideC16H21NO3[α]D20 = −29.0 (c 1.03, CH3OH)Source of chirality: (−)-(S)-phenylethylamineAbsolute configuration: (1R,5S,6R,8R)
(−)-(3aS,4R,6R,6aS)-6-Hydroxy-N-[(1S)-1-phenylethyl]hexahydro-1H-cyclopenta[c]furan-4-carboxamideC16H21NO3[α]D20 = −21.6 (c 0.9, CH3OH)Source of chirality: (−)-(S)-phenylethylamineAbsolute configuration: (3aS,4R,6R,6aS)
Journal: Tetrahedron: Asymmetry - Volume 26, Issues 12–13, 15 July 2015, Pages 608–612