| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345963 | 1500356 | 2012 | 7 صفحه PDF | دانلود رایگان |
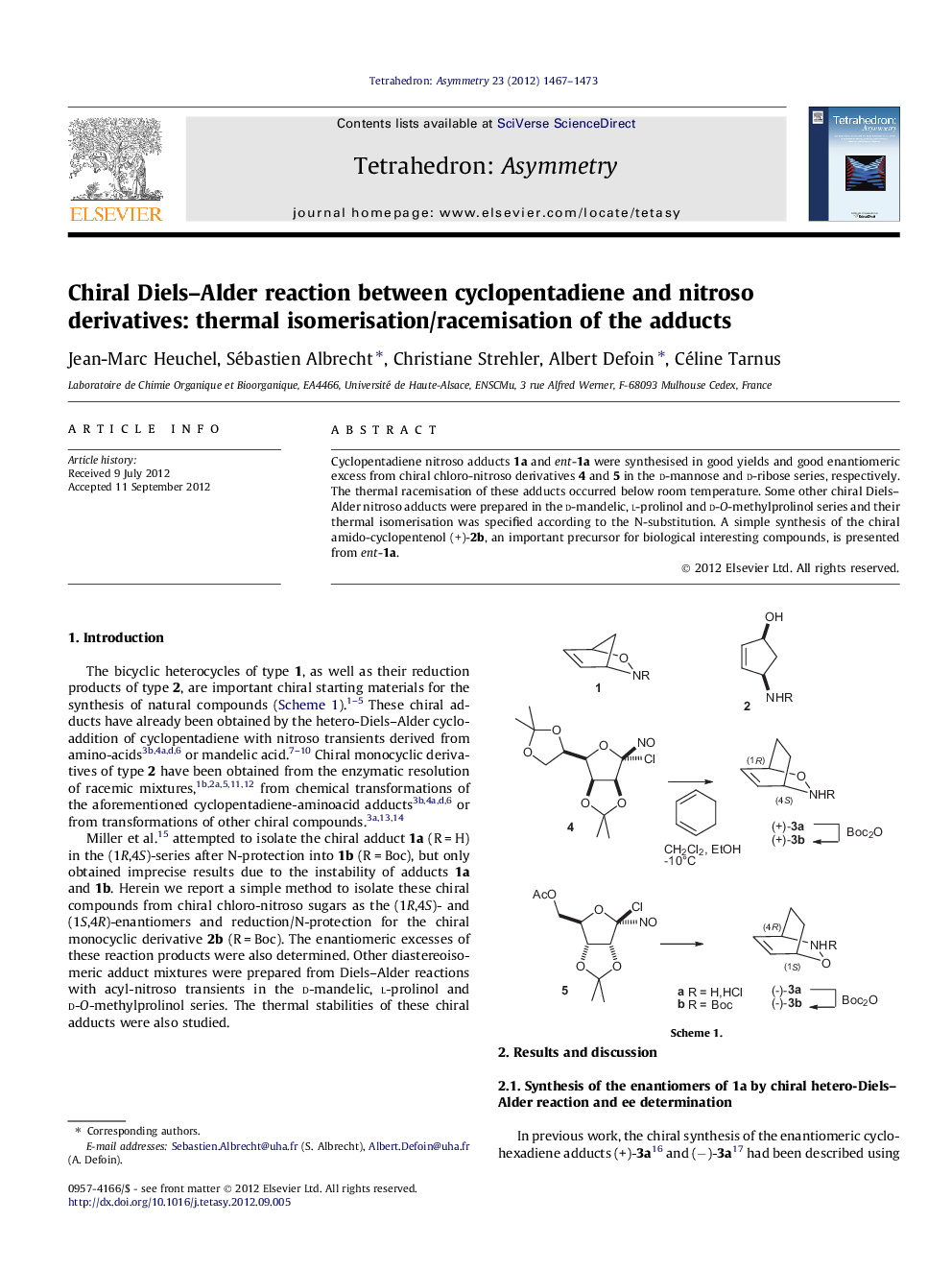
Cyclopentadiene nitroso adducts 1a and ent-1a were synthesised in good yields and good enantiomeric excess from chiral chloro-nitroso derivatives 4 and 5 in the d-mannose and d-ribose series, respectively. The thermal racemisation of these adducts occurred below room temperature. Some other chiral Diels–Alder nitroso adducts were prepared in the d-mandelic, l-prolinol and d-O-methylprolinol series and their thermal isomerisation was specified according to the N-substitution. A simple synthesis of the chiral amido-cyclopentenol (+)-2b, an important precursor for biological interesting compounds, is presented from ent-1a.
Figure optionsDownload as PowerPoint slide
(1R,4S,αR)-3-(α-Hydroxyphenylacetyl)-2-oxa-3-aza-bicyclo[2,2,1]hept-5-eneC13H14NO3de = 90%[α]D25=-78 (c 0.25, CHCl3)Source of chirality: the precursor and d-mandelic acidAbsolute configuration: (1R,4S,αR)
(1S,4R,αR)-3-(α-Hydroxyphenylacetyl)-2-oxa-3-aza-bicyclo[2,2,1]hept-5-eneC13H14NO3de = 96%[α]D25=+69 (c 0.25, CHCl3)Source of chirality: the precursor and d-mandelic acidAbsolute configuration: (1S,4R,αR)
(1S,4R)-4-tert-Butylcarbonylamino-cyclopent-2-en-1-olee = 89%[α]D=+67[α]D=+67 (c 1, CHCl3)Source of chirality: the precursorAbsolute configuration: (1S,4R)
Journal: Tetrahedron: Asymmetry - Volume 23, Issues 20–21, 15 November 2012, Pages 1467–1473