| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1345966 | 1500356 | 2012 | 6 صفحه PDF | دانلود رایگان |
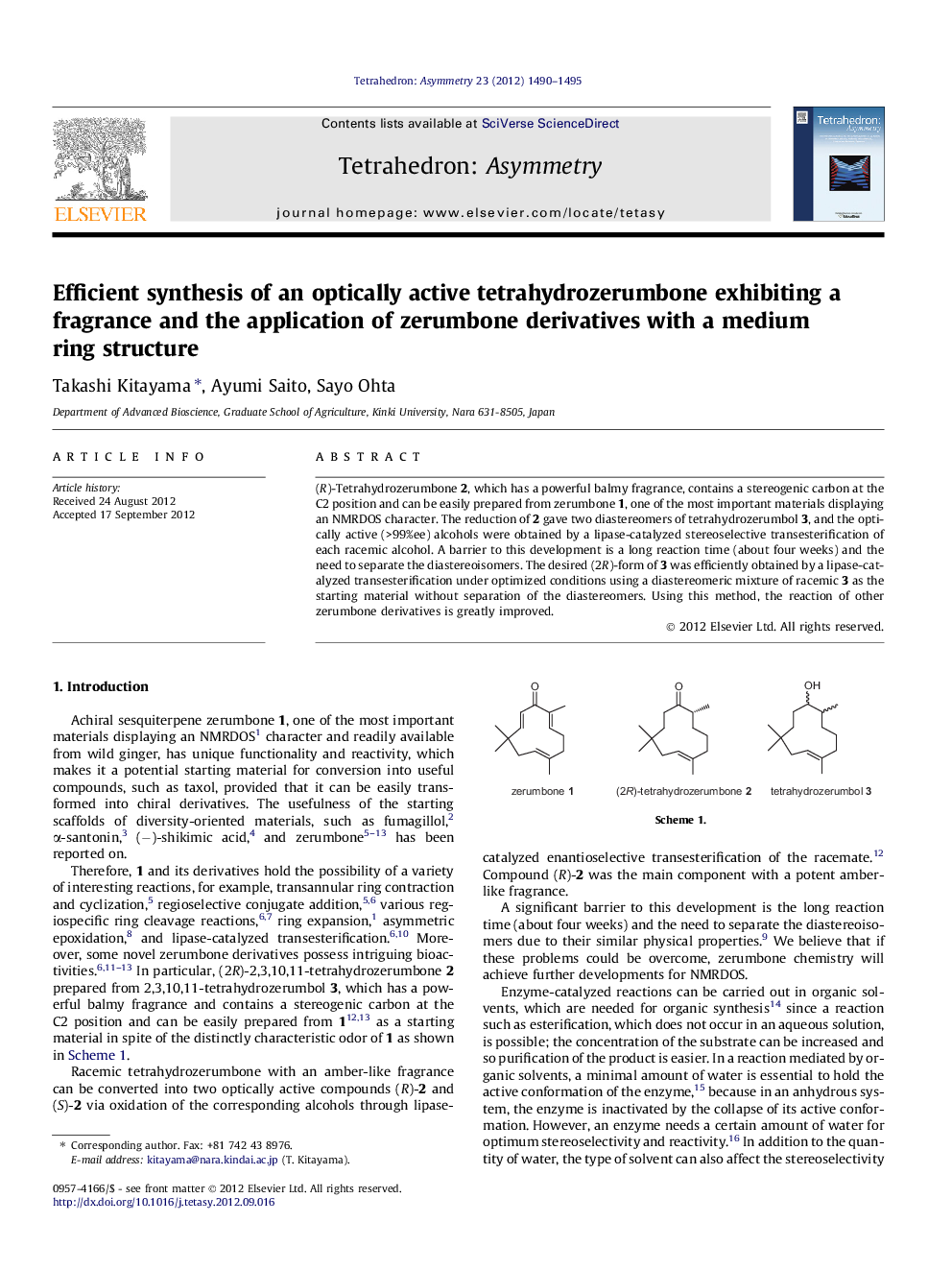
(R)-Tetrahydrozerumbone 2, which has a powerful balmy fragrance, contains a stereogenic carbon at the C2 position and can be easily prepared from zerumbone 1, one of the most important materials displaying an NMRDOS character. The reduction of 2 gave two diastereomers of tetrahydrozerumbol 3, and the optically active (>99%ee) alcohols were obtained by a lipase-catalyzed stereoselective transesterification of each racemic alcohol. A barrier to this development is a long reaction time (about four weeks) and the need to separate the diastereoisomers. The desired (2R)-form of 3 was efficiently obtained by a lipase-catalyzed transesterification under optimized conditions using a diastereomeric mixture of racemic 3 as the starting material without separation of the diastereomers. Using this method, the reaction of other zerumbone derivatives is greatly improved.
Figure optionsDownload as PowerPoint slide
(2R)-2,6,9,9-Tetramethyl-6-cycloundecen-1-oneC15H26OEe = 99%[α]D23.5=-53.6 (c 0.85, CHCl3)Source of chirality: lipase resolutionAbsolute configuration: (2R)
Journal: Tetrahedron: Asymmetry - Volume 23, Issues 20–21, 15 November 2012, Pages 1490–1495