| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346003 | 1500358 | 2012 | 16 صفحه PDF | دانلود رایگان |
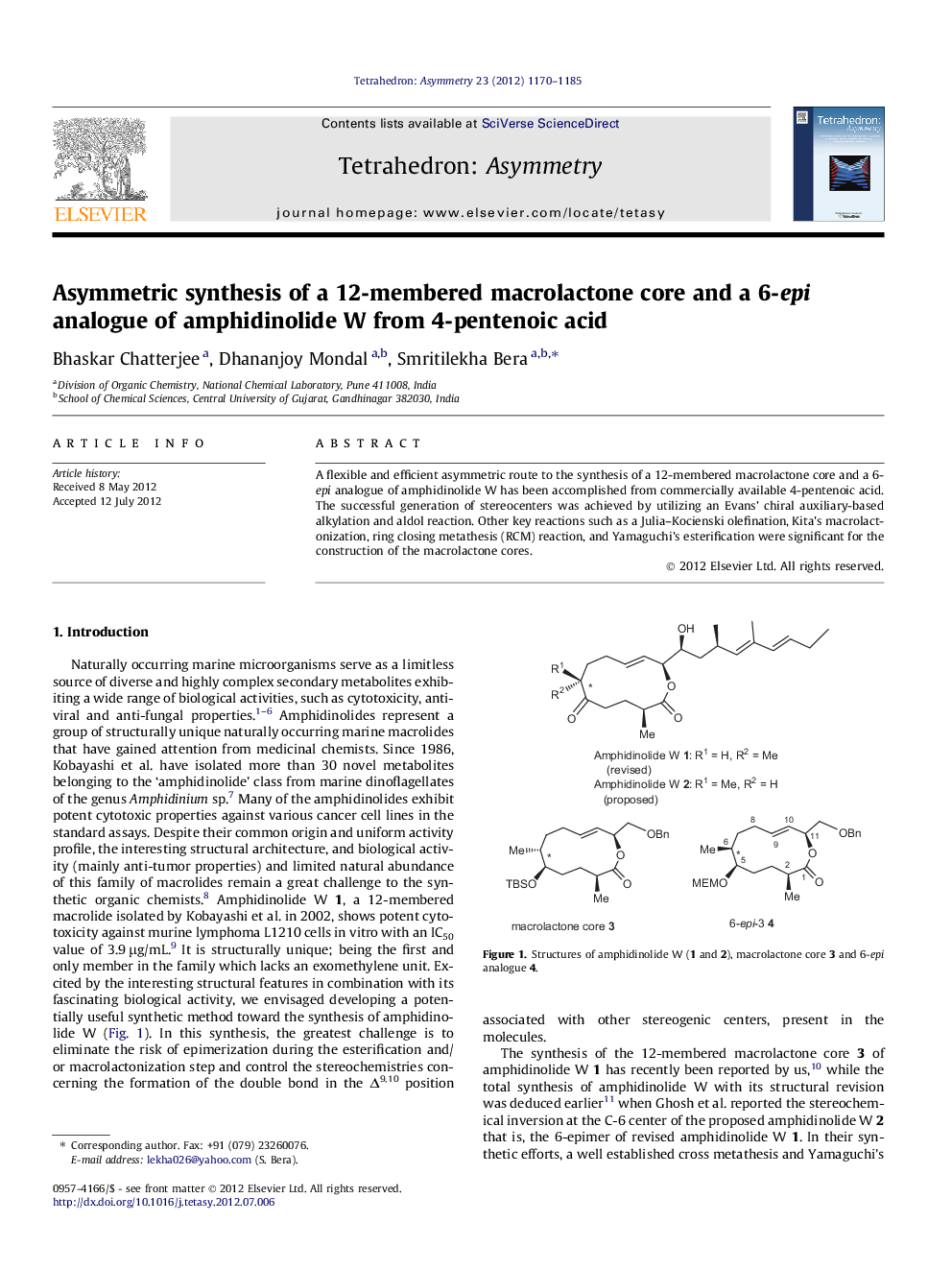
A flexible and efficient asymmetric route to the synthesis of a 12-membered macrolactone core and a 6-epi analogue of amphidinolide W has been accomplished from commercially available 4-pentenoic acid. The successful generation of stereocenters was achieved by utilizing an Evans’ chiral auxiliary-based alkylation and aldol reaction. Other key reactions such as a Julia–Kocienski olefination, Kita’s macrolactonization, ring closing metathesis (RCM) reaction, and Yamaguchi’s esterification were significant for the construction of the macrolactone cores.
Figure optionsDownload as PowerPoint slide
(S)-4-Benzyl-3-((S)-2-methylpent-4-enoyl)oxazolidin-2-oneC16H19NO3[α]D25=+77.2 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (4S,7S)
(2S)-2-Methylpent-4-enoic acidC6H10O2[α]D25=+9.9 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2S)
(S)-4-Benzyl-3-((2S,3R,6S)-7-(benzyloxy)-2-(but-3-enyl)-3-hydroxy-6-methylheptanoyl)oxazolidin-2-oneC29H37NO5[α]D25=+29.0 (c 1.2, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (4S,7S,8R,11S)
(2R,3R,6S)-7-(Benzyloxy)-2-(but-3-enyl)-6-methylheptane-1,3-diolC19H30O3[α]D25=+7.5 (c 0.9, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2R,3R,6S)
(2S,5R,6S)-1-(Benzyloxy)-2,6-dimethyldec-9-en-5-olC19H30O2[α]D25=+5.0 (c 1.5, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2R,5R,6S)
(2S,5R,6S)-5-((2-Methoxyethoxy)methoxy)-2,6-dimethyldec-9-enoic acidC16H30O5[α]D25=+23.2 (c 0.8, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,5R,6S)
(2S,5R,6S)-((S)-1-(Benzyloxy)but-3-en-2-yl)-5-((2-methoxyethoxy)methoxy)-2,6-dimethyldec-9-enoateC27H42O6[α]D25=+13.2 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,5R,6S)
(S)-4-Benzyl-3-((2S,3R,6S)-7-(benzyloxy)-3-hydroxy-2,6-dimethylheptanoyl)oxazolidin-2-oneC26H33NO5[α]D25=+42.0 (c 1.9, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (4S,7S,8R,11S)
(2R,3R,6S)-7-(Benzyloxy)-3-(methoxymethoxy)-2,6-dimethylheptan-1-olC18H30O4[α]D25=-28.3 (c 1.2, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2R,3R,6S)
(S)-4-Benzyl-3-((2S,3R,6S)-7-(benzyloxy)-3-(tert-butyldimethylsilyloxy)-2,6-dimethylheptanoyl)oxazolidin-2-oneC32H47NO5Si[α]D25=+28.4 (c 1.1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (4S,7S,8R,11S)
(2R,3R,6S)-7-(Benzyloxy)-3-(tert-butyldimethylsilyloxy)-2,6-dimethylheptan-1-olC22H40O3Si[α]D25=-1.95 (c 0.8, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2R,3R,6S)
((2S,5R,6R,9E)-1-(Benzyloxy)-10-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)-2,6-dimethyldec-9-en-5-yloxy)(tert-butyl)dimethylsilaneC30H52O4Si[α]D25=+17.9 (c 2.2, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,5R,6R,9E,11S)
(2S,7R,8R,11S,3E)-8-(tert-Butyldimethylsilyloxy)-7,11-dimethyldodec-3-en-1,2,12-triolC20H42O4Si[α]D25=+3.8 (c 1.2, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,7R,8R,11S,3E)
(2S,5R,6R,11S,9E)-12-(Benzyloxy)-5-(tert-butyldimethylsilyloxy)-2,6-dimethyldodec-9-en-1,11-diolC27H48O4Si[α]D25=+10.8 (c 1.3, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,5R,6R,11S,9E)
(2S,5R,6R,11S,9E)-12-(Benzyloxy)-5-(tert-butyldimethylsilyloxy)-11-hydroxy-2,6-dimethyldodec-9-enoic acidC27H46O5Si[α]D25=+20.0 (c 1.1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,5R,6R,11S,9E)
(3S,6R,7R,12S,10E)-12-(Benzyloxymethyl)-6-(tert-butyldimethylsilyloxy)-3,7-dimethyloxacyclododec-10-en-2-oneC27H44O4Si[α]D25=+29.0 (c 0.6, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3S,6R,7R,12S,10E)
Journal: Tetrahedron: Asymmetry - Volume 23, Issues 15–16, 31 August 2012, Pages 1170–1185