| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346005 | 1500358 | 2012 | 8 صفحه PDF | دانلود رایگان |
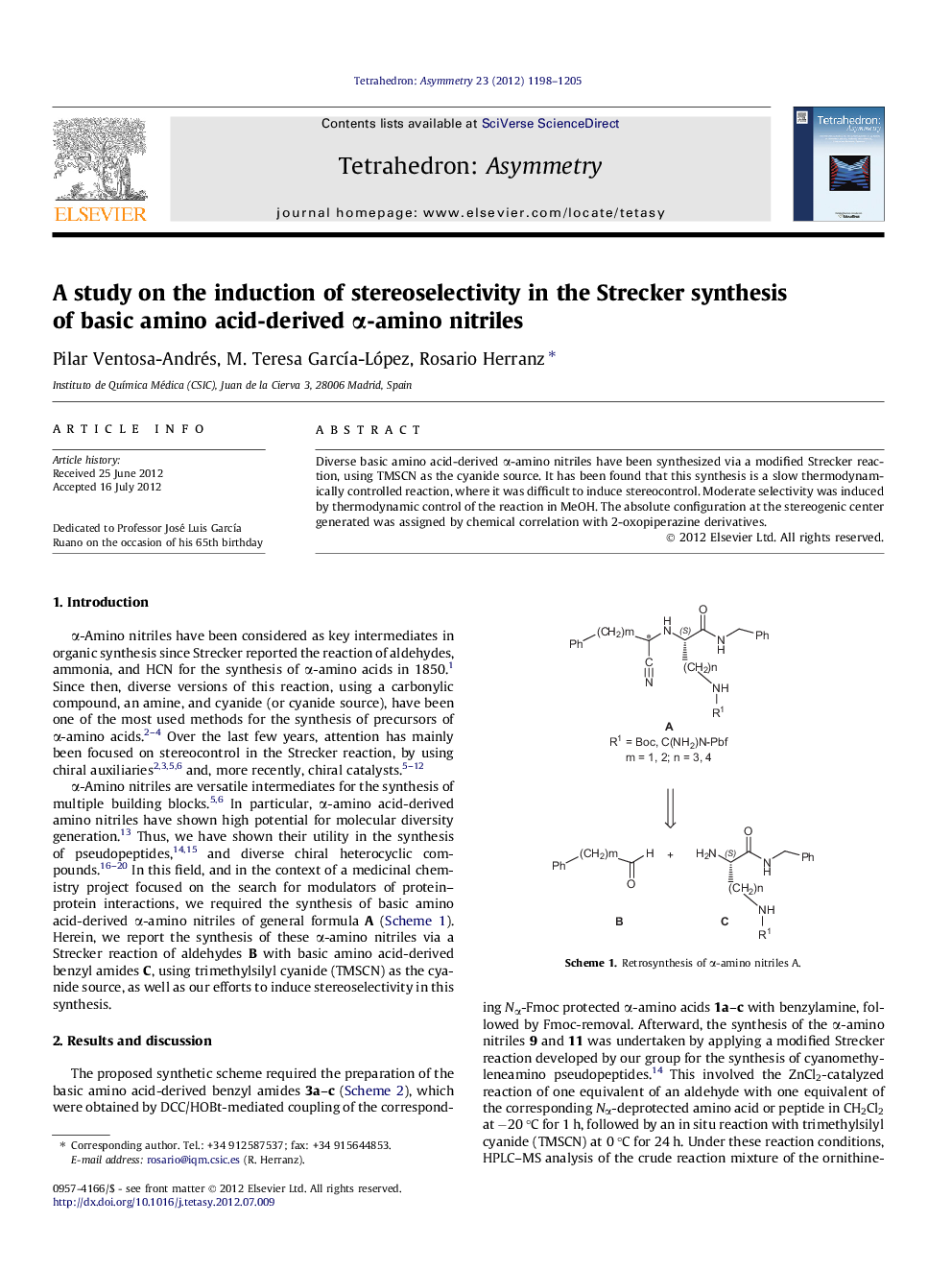
Diverse basic amino acid-derived α-amino nitriles have been synthesized via a modified Strecker reaction, using TMSCN as the cyanide source. It has been found that this synthesis is a slow thermodynamically controlled reaction, where it was difficult to induce stereocontrol. Moderate selectivity was induced by thermodynamic control of the reaction in MeOH. The absolute configuration at the stereogenic center generated was assigned by chemical correlation with 2-oxopiperazine derivatives.
Figure optionsDownload as PowerPoint slide
tert-Butyl ((S)-5-(benzylamino)-4-(((R)-1-cyano-2-phenylethyl)amino)-5-oxopentyl)carbamateC26H34N4O3[α]D20=+7.5 (c 1, MeOH)Source of chirality: Fmoc-Orn(Boc)-OH and chromatographic diastereoisomer resolutionAbsolute configuration: (S,R)
tert-Butyl ((S)-5-(benzylamino)-4-(((S)-1-cyano-2-phenylethyl)amino)-5-oxopentyl)carbamateC26H34N4O3[α]D20=+7.5+21.7 (c 0.9, MeOH)Source of chirality: Fmoc-Orn(Boc)-OH and chromatographic diastereoisomer resolutionAbsolute configuration: (S,S)
tert-Butyl ((S)-6-(benzylamino)-5-(((R)-1-cyano-2-phenylethyl)amino)-6-oxohexyl)carbamateC27H36N4O3[α]D20=+7.5= +9.2 (c 1.2, MeOH)Source of chirality: Fmoc-Lys(Boc)-OH and chromatographic diastereoisomer resolutionAbsolute configuration: (S,R)
tert-Butyl ((S)-6-(benzylamino)-5-(((S)-1-cyano-2-phenylethyl)amino)-6-oxohexyl)carbamateC27H36N4O3[α]D20=+27.1 (c 1, MeOH)Source of chirality: Fmoc-Lys(Boc)-OH and chromatographic diastereoisomer resolutionAbsolute configuration: (S,S)
(S)-Methyl 5-((tert-butoxycarbonyl)amino)-2-(((R)-1-cyano-2-phenylethyl)amino)pentanoateC20H29N3O4[α]D20=+7.5 (c 1, MeOH)Source of chirality: H-Orn(Boc)-OMe and chromatographic diastereoisomer resolutionAbsolute configuration: (S,R)
(S)-Methyl 5-((tert-butoxycarbonyl)amino)-2-(((S)-1-cyano-2-phenylethyl)amino)pentanoateC20H29N3O4[α]D20=-22.9 (c 1.2, MeOH)Source of chirality: H-Orn(Boc)-OMe and chromatographic diastereoisomer resolutionAbsolute configuration: (S,S)
Journal: Tetrahedron: Asymmetry - Volume 23, Issues 15–16, 31 August 2012, Pages 1198–1205