| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346191 | 980247 | 2013 | 4 صفحه PDF | دانلود رایگان |
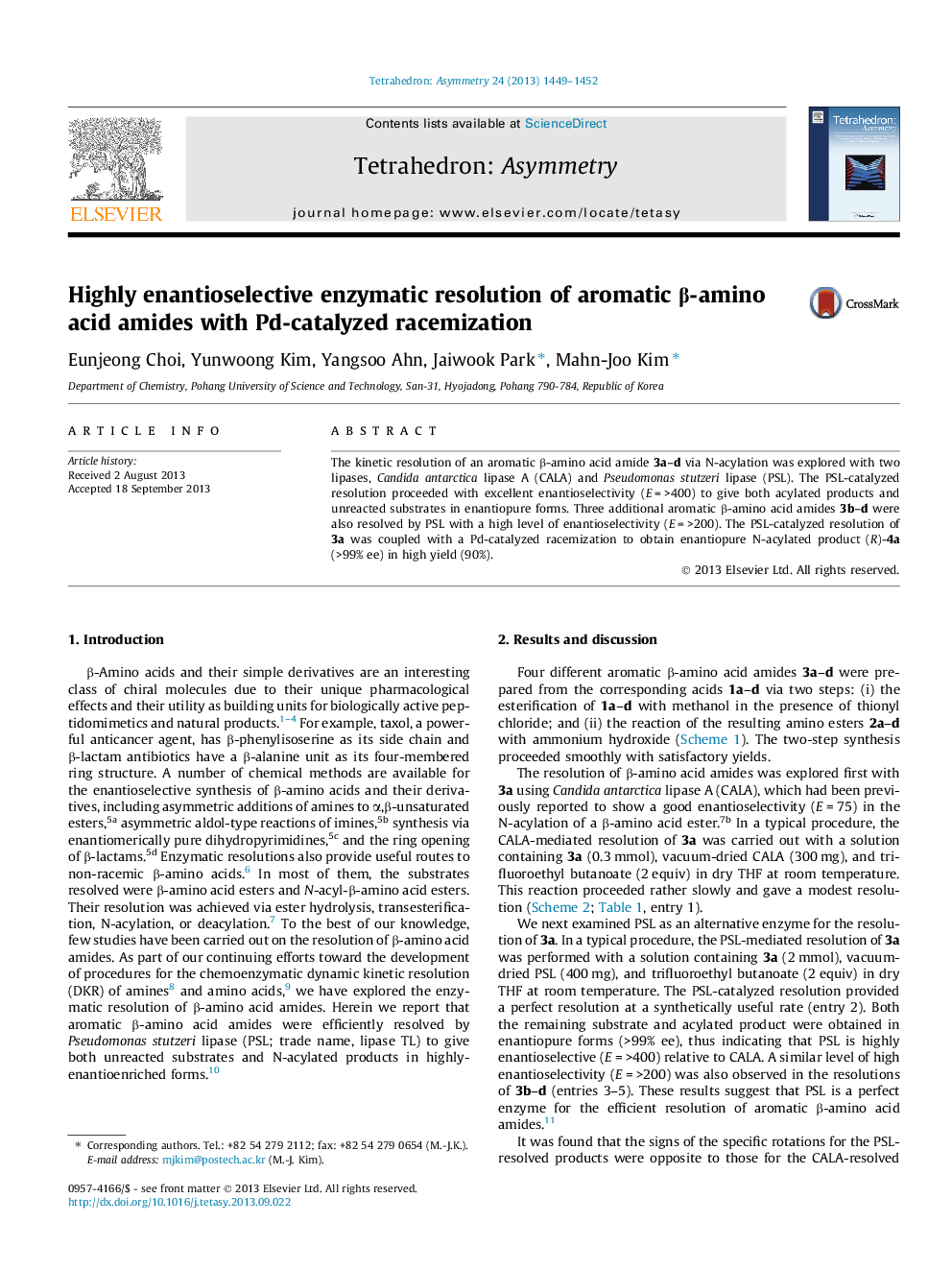
The kinetic resolution of an aromatic β-amino acid amide 3a–d via N-acylation was explored with two lipases, Candida antarctica lipase A (CALA) and Pseudomonas stutzeri lipase (PSL). The PSL-catalyzed resolution proceeded with excellent enantioselectivity (E = >400) to give both acylated products and unreacted substrates in enantiopure forms. Three additional aromatic β-amino acid amides 3b–d were also resolved by PSL with a high level of enantioselectivity (E = >200). The PSL-catalyzed resolution of 3a was coupled with a Pd-catalyzed racemization to obtain enantiopure N-acylated product (R)-4a (>99% ee) in high yield (90%).
Figure optionsDownload as PowerPoint slide
(S)-3-Amino-3-phenylpropanamideC9H12N2OEe ⩾ 99%[α]D25=-51 (c 0.5, THF)Source of chirality: enzymatic resolutionAbsolute configuration: (S)
(S)-3-Amino-3-(4-fluorophenyl)propanamideC9H11FN2OEe ⩾ 99%[α]D25=-41.5 (c 0.24, CHCl3)Source of chirality: enzymatic resolutionAbsolute configuration: (S)
(S)-3-Amino-3-p-tolylpropanamideC10H14N2OEe = 96%[α]D25=-43 (c 0.25, CHCl3)Source of chirality: enzymatic resolutionAbsolute configuration: (S)
(S)-3-Amino-3-(4-methoxyphenyl)propanamideC10H14N2O2Ee = 88%[α]D25=-33 (c 0.5, CHCl3)Source of chirality: enzymatic resolutionAbsolute configuration: (S)
(R)-N-(3-Amino-3-oxo-1-phenylpropyl)butyramideC13H18N2O2Ee ⩾ 99%[α]D25=+59 (c 0.1, THF)Source of chirality: enzymatic resolutionAbsolute configuration: (R)
(R)-N-(3-Amino-1-(4-fluorophenyl)-3-oxopropyl)butyramideC13H17FN2O2Ee = 96%[α]D25=+118 (c 0.1, MeOH)Source of chirality: enzymatic resolutionAbsolute configuration: (R)
(R)-N-(3-Amino-3-oxo-1-p-tolylpropyl)butyramideC14H20N2O2Ee = 99%[α]D25=+92 (c 0.2, MeOH)Source of chirality: enzymatic resolutionAbsolute configuration: (R)
(R)-N-(3-Amino-1-(4-methoxyphenyl)-3-oxopropyl)butyramideC14H20N2O3Ee ⩾ 99%[α]D25=+85 (c 0.1, MeOH)Source of chirality: enzymatic resolutionAbsolute configuration: (R)
Journal: Tetrahedron: Asymmetry - Volume 24, Issue 23, 15 December 2013, Pages 1449–1452