| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346213 | 1500362 | 2011 | 9 صفحه PDF | دانلود رایگان |
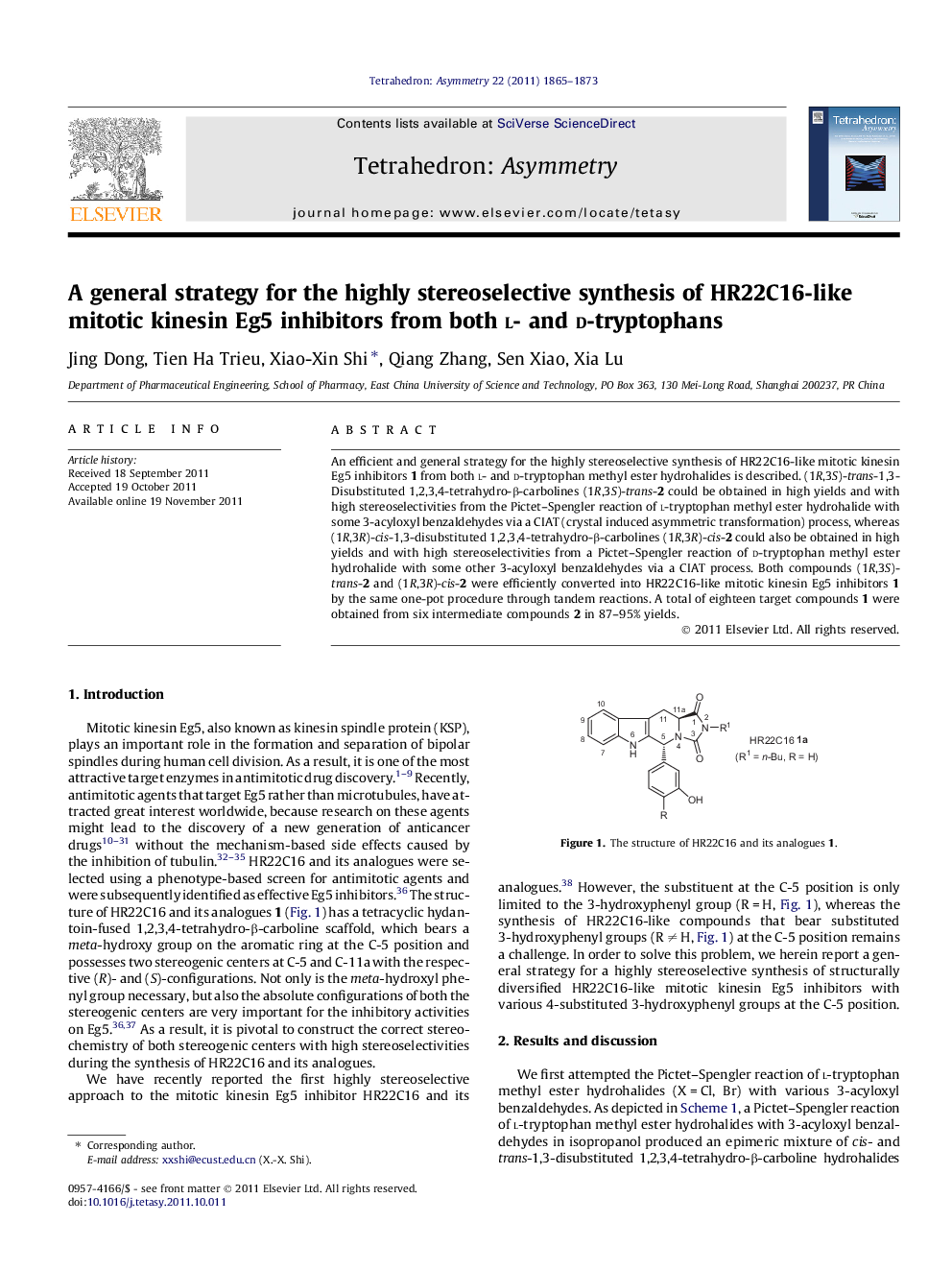
An efficient and general strategy for the highly stereoselective synthesis of HR22C16-like mitotic kinesin Eg5 inhibitors 1 from both l- and d-tryptophan methyl ester hydrohalides is described. (1R,3S)-trans-1,3-Disubstituted 1,2,3,4-tetrahydro-β-carbolines (1R,3S)-trans-2 could be obtained in high yields and with high stereoselectivities from the Pictet–Spengler reaction of l-tryptophan methyl ester hydrohalide with some 3-acyloxyl benzaldehydes via a CIAT (crystal induced asymmetric transformation) process, whereas (1R,3R)-cis-1,3-disubstituted 1,2,3,4-tetrahydro-β-carbolines (1R,3R)-cis-2 could also be obtained in high yields and with high stereoselectivities from a Pictet–Spengler reaction of d-tryptophan methyl ester hydrohalide with some other 3-acyloxyl benzaldehydes via a CIAT process. Both compounds (1R,3S)-trans-2 and (1R,3R)-cis-2 were efficiently converted into HR22C16-like mitotic kinesin Eg5 inhibitors 1 by the same one-pot procedure through tandem reactions. A total of eighteen target compounds 1 were obtained from six intermediate compounds 2 in 87–95% yields.
Figure optionsDownload as PowerPoint slide
(5R,11aS)-2-Propyl-5-(3-hydroxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC22H21N3O3[α]D20=-202.4 (c 0.5, CHCl3)Source of chirality: l-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-Hydroxyethyl-5-(3-hydroxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC21H19N3O4[α]D20=-238.1 (c 0.6, CH3OH)Source of chirality: l-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-Benzyl-5-(3-hydroxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC26H21N3O3[α]D20=-162.5 (c 0.8, CHCl3)Source of chirality: l-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-Isopropyl-5-(3-hydroxy-4-methoxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC23H23N3O4[α]D20=-231.9 (c 0.6, CHCl3)Source of chirality: l-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-(5-Azido-pentyl)-5-(3-hydroxy-4-methoxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC25H26N6O4[α]D20=-205.4 (c 0.8, CHCl3)Source of chirality: l-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-Phenethyl-5-(3-hydroxy-4-methoxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC28H25N3O4[α]D20=-192.4 (c 0.6, CHCl3)Source of chirality: l-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-Isopropyl-5-(3,4-dihydroxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC22H21N3O4[α]D20=-192.5 (c 0.6, CHCl3)Source of chirality: l-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-(5-Azido-pentyl)-5-(3-hydroxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC24H24N6O3[α]D20=-204.6 (c 0.3, CHCl3)Source of chirality: d-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-Isopropyl-5-(3-hydroxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC22H21N3O3[α]D20=-223.8 (c 0.8, CHCl3)Source of chirality: d-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-Phenethyl-5-(3-hydroxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC27H23N3O3[α]D20=-213.1 (c 0.4, CHCl3)Source of chirality: d-tryptophanAbsolute configuration: (5R,11aS)
(1R,3S)-Methyl 1-(3-(benzoyloxy)phenyl)-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylateC26H22N2O4[α]D20=-33.7 (c 0.8, CHCl3)Source of chirality: l-tryptophanAbsolute configuration: (1R,3S)
(5R,11aS)-2-Propyl-5-(3-hydroxy-4-methoxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC23H23N3O4[α]D20=-233.7 (c 0.6, CHCl3)Source of chirality: d-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-Butyl-5-(3-hydroxy-4-methoxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC24H25N3O4[α]D20=-226.9 (c 0.7, CHCl3)Source of chirality: d-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-Hydroxyethyl-5-(3-hydroxy-4-methoxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC22H21N3O5[α]D20=-230.7 (c 1.0, CH3OH)Source of chirality: d-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-Benzyl-5-(3-hydroxy-4-methoxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC27H23N3O4[α]D20=-191.3 (c 1.2, CHCl3)Source of chirality: d-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-(5-Azido-pentyl)-5-(3,4-dihydroxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC24H24N6O4[α]D20=-180.9 (c 0.6, CH3OH)Source of chirality: d-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-Hydroxyethyl-5-(3,4-dihydroxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC21H19N3O5[α]D20=-219.6 (c 0.5, CH3OH)Source of chirality: d-tryptophanAbsolute configuration: (5R,11aS)
(5R,11aS)-2-Benzyl-5-(3,4-dihydroxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC26H21N3O4[α]D20=-179.0 (c 0.6, CHCl3)Source of chirality: d-tryptophanAbsolute configuration: (5R,11aS)
(1R,3R)-Methyl 1-(3-acetoxyphenyl)-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylateC21H20N2O4[α]D20=+7.1 (c 3.0, CHCl3)Source of chirality: d-tryptophanAbsolute configuration: (1R,3R)
(1R,3S)-Methyl 1-(3-benzoyloxy-4-methoxyphenyl)-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylateC27H24N2O5[α]D20=-36.3 (c 1.0, CHCl3)Source of chirality: l-tryptophanAbsolute configuration: (1R,3S)
(1R,3S)-Methyl 1-(3-acetoxy-4-methoxyphenyl)-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylateC22H22N2O5[α]D20=-29.8 (c 1.0, CHCl3)Source of chirality: l-tryptophanAbsolute configuration: (1R,3S)
(1R,3R)-Methyl 1-(3-acetoxy-4-methoxyphenyl)-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylateC22H22N2O5[α]D20=+9.5 (c 5.0, CHCl3)Source of chirality: d-tryptophanAbsolute configuration: (1R,3R)
(1R,3S)-Methyl 1-(3,4-diacetoxyphenyl)-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylateC23H22N2O6[α]D20=-38.5 (c 0.6, CHCl3)Source of chirality: l-tryptophanAbsolute configuration: (1R,3S)
(1R,3R)-Methyl 1-(3,4-diacetoxyphenyl)-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylateC23H22N2O6[α]D20=+5.6 (c 7.0, CHCl3)Source of chirality: d-tryptophanAbsolute configuration: (1R,3R)
(1R,3S)-Methyl 1-(3,4-dibenzoyloxyphenyl)-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylateC33H26N2O6[α]D20=-47.5 (c 0.4, CHCl3)Source of chirality: l-tryptophanAbsolute configuration: (1R,3S)
(5R,11aS)-2-Butyl-5-(3-hydroxyphenyl)-6H-1,2,3,5,11,11a-hexahydro-imidazo[1,5-b]-β-carboline-1,3-dioneC23H23N3O3[α]D20=-231.5 (c 0.3, CHCl3)Source of chirality: l-tryptophanAbsolute configuration: (5R,11aS)
Journal: Tetrahedron: Asymmetry - Volume 22, Issues 20–22, 30 November 2011, Pages 1865–1873