| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346219 | 1500362 | 2011 | 5 صفحه PDF | دانلود رایگان |
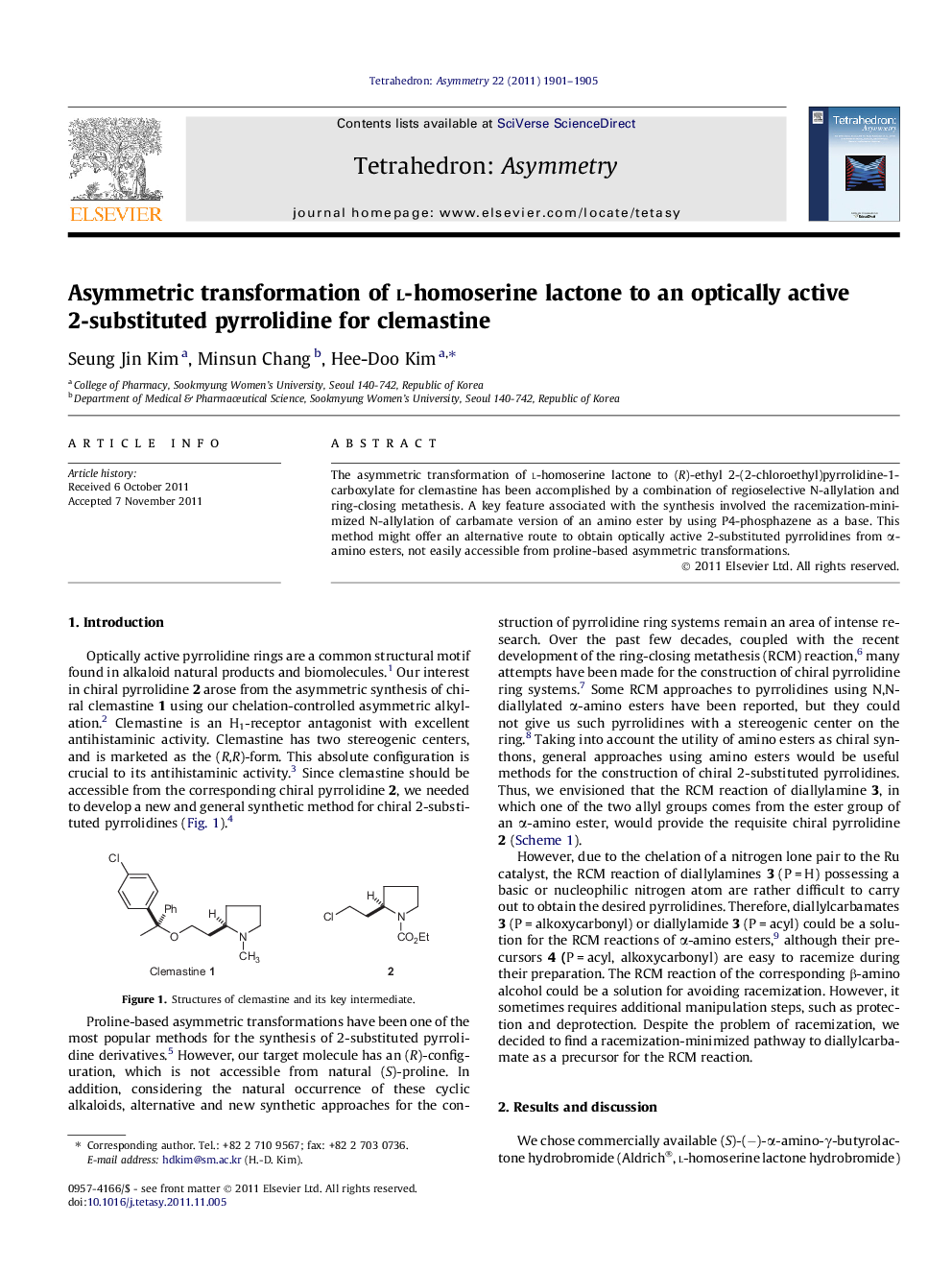
The asymmetric transformation of l-homoserine lactone to (R)-ethyl 2-(2-chloroethyl)pyrrolidine-1-carboxylate for clemastine has been accomplished by a combination of regioselective N-allylation and ring-closing metathesis. A key feature associated with the synthesis involved the racemization-minimized N-allylation of carbamate version of an amino ester by using P4-phosphazene as a base. This method might offer an alternative route to obtain optically active 2-substituted pyrrolidines from α-amino esters, not easily accessible from proline-based asymmetric transformations.
Figure optionsDownload as PowerPoint slide
(S)-Ethyl allyl(2-oxotetrahydrofuran-3-yl)carbamateC10H15NO4Ee > 98%[α]D25=-4.8 (c 1.0, chloroform)Source of chirality: l-homoserine lactoneAbsolute configuration: (S)
(S,E)-Ethyl 4-(allyl(ethoxycarbonyl)amino)-6-hydroxyhex-2-enoateC14H23NO5Ee = 96%[α]D25=-37.7 (c 1.4, chloroform)Source of chirality: l-homoserine lactoneAbsolute configuration: (S)
(S)-Ethyl 2-(2-hydroxyethyl)-2,5-dihydro-1H-pyrrole-1-carboxylateC9H15NO3Ee = 95%[α]D23=+104.6 (c 1.3, CHCl3)Source of chirality: l-homoserine lactoneAbsolute configuration: (S)
(R)-Ethyl 2-(2-hydroxyethyl)pyrrolidine-1-carboxylateC9H17NO3Ee = 95%[α]D25=+4.7 (c 1.0, CHCl3)Source of chirality: l-homoserine lactoneAbsolute configuration: (R)
(R)-Ethyl 2-(2-chloroethyl)pyrrolidine-1-carboxylateC9H16ClNO2Ee = 95%[α]D25=+32.5 (c 1.4, CHCl3)Source of chirality: l-homoserine lactoneAbsolute configuration: (R)
Journal: Tetrahedron: Asymmetry - Volume 22, Issues 20–22, 30 November 2011, Pages 1901–1905