| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346220 | 1500362 | 2011 | 12 صفحه PDF | دانلود رایگان |
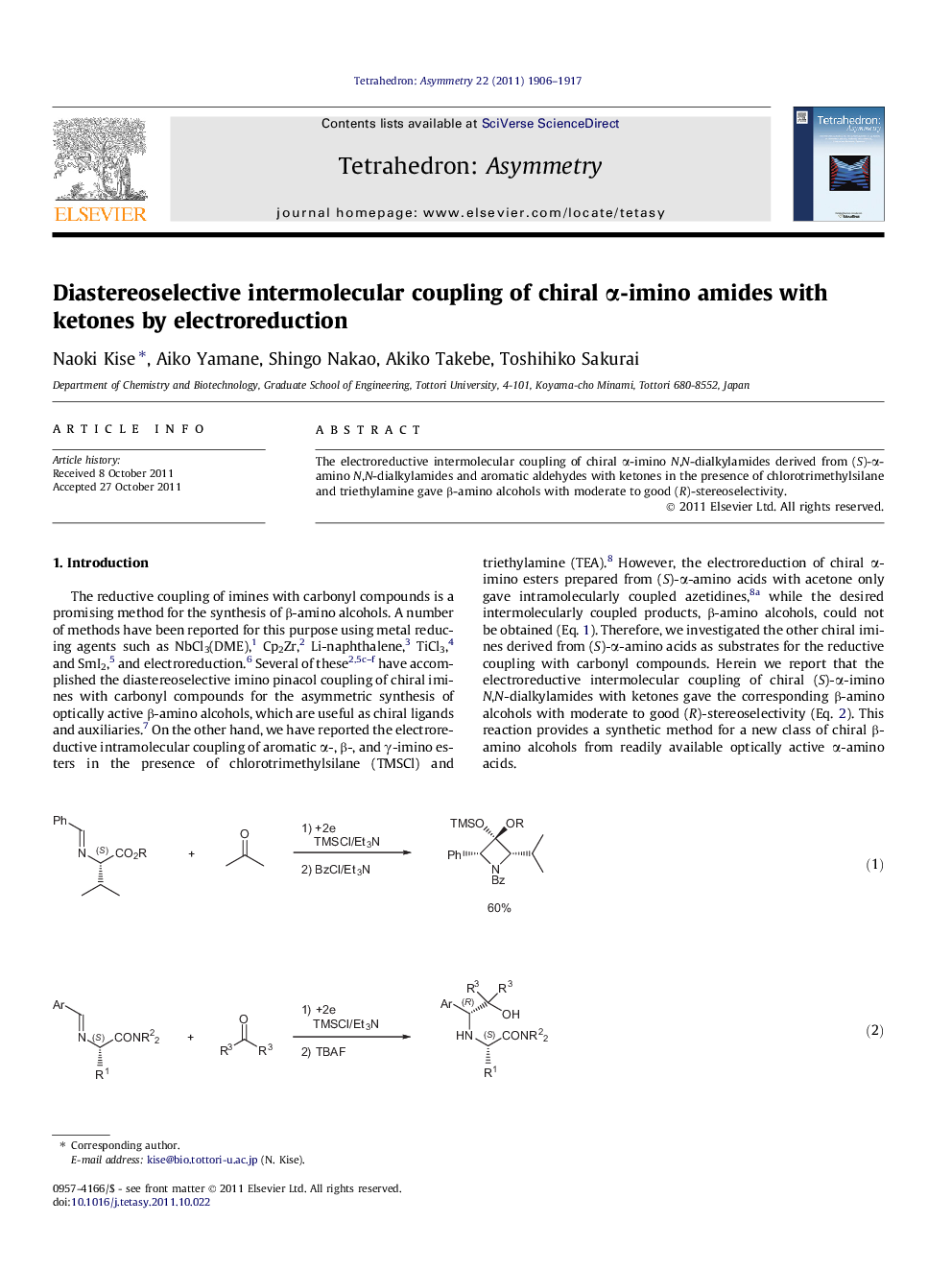
The electroreductive intermolecular coupling of chiral α-imino N,N-dialkylamides derived from (S)-α-amino N,N-dialkylamides and aromatic aldehydes with ketones in the presence of chlorotrimethylsilane and triethylamine gave β-amino alcohols with moderate to good (R)-stereoselectivity.
Figure optionsDownload as PowerPoint slide
(S)-2-((R)-2-Hydroxy-2-methyl-1-phenylpropylamino)-N,N,3-trimethylbutanamideC17H28N2O2[α]D24=-76.0 (c 1.05, CHCl3)Source of chirality: (S)-valineAbsolute configuration: (S,R)
(S)-2-((S)-2-Hydroxy-2-methyl-1-phenylpropylamino)-N,N,3-trimethylbutanamideC17H28N2O2[α]D23=+44.3 (c 1.40, CHCl3)Source of chirality: (S)-valineAbsolute configuration: (S,S)
(S)-N,N-diethyl-2-((R)-2-hydroxy-2-methyl-1-phenylpropylamino)-3-methylbutanamideC19H32N2O2[α]D23=+84.8 (c 1.09, CHCl3)Source of chirality: (S)-valineAbsolute configuration: (S,R)
(S)-2-((R)-2-Hydroxy-2-methyl-1-phenylpropylamino)-3-methyl-1-(piperidin-1-yl)butan-1-oneC20H32N2O2[α]D22=-73.7 (c 1.00, CHCl3)Source of chirality: (S)-valineAbsolute configuration: (S,R)
(S)-2-((S)-2-Hydroxy-2-methyl-1-phenylpropylamino)-3-methyl-1-(piperidin-1-yl)butan-1-oneC20H32N2O2[α]D23=+57.7 (c 0.92, CHCl3).Source of chirality: (S)-valineAbsolute configuration: (S,S)
(2S,3S)-2-((R)-2-Hydroxy-2-methyl-1-phenylpropylamino)-N,N,3-trimethylpentanamideC18H30N2O2[α]D23=+72.0 (c 1.00, CHCl3).Source of chirality: (S)-isoleucineAbsolute configuration: (S,S,R)
(2S,3S)-2-((S)-2-Hydroxy-2-methyl-1-phenylpropylamino)-N,N,3-trimethylpentanamideC18H30N2O2[α]D23=+40.4 (c 1.05, CHCl3).Source of chirality: (S)-isoleucineAbsolute configuration: (S,S,S)
(2S,3S)-N,N-Diethyl-2-((R)-2-hydroxy-2-methyl-1-phenylpropylamino)-3-methylpentanamideC20H34N2O2[α]D24=-73.1 (c 1.50, CHCl3).Source of chirality: (S)-isoleucineAbsolute configuration: (S,S,R)
(2S,3S)-N,N-Diethyl-2-((S)-2-hydroxy-2-methyl-1-phenylpropylamino)-3-methylpentanamideC20H34N2O2[α]D24=+27.6 (c 1.14, CHCl3).Source of chirality: (S)-isoleucineAbsolute configuration: (S,S,S)
(S)-2-((R)-2-Hydroxy-2-methyl-1-phenylpropylamino)-N,N,4-trimethylpentanamideC18H30N2O2[α]D21=-78.7 (c 1.01, CHCl3).Source of chirality: (S)-isoleucineAbsolute configuration: (S,R)
(S)-2-((R)-2-Hydroxy-2-methyl-1-phenylpropylamino)-N,N-dimethyl-3-phenylpropanamideC21H28N2O2[α]D23=+12.0 (c 1.07, CHCl3).Source of chirality: (S)-phenylalanineAbsolute configuration: (S,R)
(S)-2-((S)-2-Hydroxy-2-methyl-1-phenylpropylamino)-N,N-dimethyl-3-phenylpropanamideC21H28N2O2[α]D25=+109 (c 1.03, CHCl3).Source of chirality: (S)-phenylalanineAbsolute configuration: (S,S)
(S)-Methyl 6-(dimethylamino)-5-((R)-2-hydroxy-2-methyl-1-phenylpropylamino)-6-oxohexanoateC19H30N2O4[α]D23=-58.7 (c 1.73, CHCl3).Source of chirality: (S)-glutamic acidAbsolute configuration: (S,R)
(S)-Methyl 6-(dimethylamino)-5-((S)-2-hydroxy-2-methyl-1-phenylpropylamino)-6-oxohexanoateC19H30N2O4[α]D23=+31.4 (c 1.14, CHCl3).Source of chirality: (S)-glutamic acidAbsolute configuration: (S,S)
(S)-N,N-Diethyl-2-((R)-2-hydroxy-1-(4-methoxyphenyl)-2-methylpropylamino)-3-methylbutanamideC20H34N2O3[α]D24=-90.0 (c 1.03, CHCl3).Source of chirality: (S)-valineAbsolute configuration: (S,R)
(S)-N,N-Diethyl-2-((S)-2-hydroxy-1-(4-methoxyphenyl)-2-methylpropylamino)-3-methylbutanamideC20H34N2O3[α]D22=+56.0 (c 1.04, CHCl3).Source of chirality: (S)-valineAbsolute configuration: (S,S)
(S)-N,N-Diethyl-2-((R)-2-hydroxy-1-(3-methoxyphenyl)-2-methylpropylamino)-3-methylbutanamideC20H34N2O3[α]D25=-85.0 (c 1.01, CHCl3).Source of chirality: (S)-valineAbsolute configuration: (S,R)
(S)-N,N-Diethyl-2-((S)-2-hydroxy-1-(3-methoxyphenyl)-2-methylpropylamino)-3-methylbutanamideC20H34N2O3[α]D19=+49.6 (c 1.02, CHCl3).Source of chirality: (S)-valineAbsolute configuration: (S,S)
(S)-N,N-Diethyl-2-((R)-2-hydroxy-1-(4-cyanophenyl)-2-methylpropylamino)-3-methylbutanamideC20H31N3O2[α]D23=-92.3 (c 1.11, CHCl3).Source of chirality: (S)-valineAbsolute configuration: (S,R)
(S)-N,N-Diethyl-2-((S)-2-hydroxy-1-(4-cyanophenyl)-2-methylpropylamino)-3-methylbutanamideC20H31N3O2[α]D21=+42.0 (c 1.05, CHCl3).Source of chirality: (S)-valineAbsolute configuration: (S,S)
(S)-N,N-Diethyl-2-((R)-2-hydroxy-2-methyl-1-(naphthalen-1-yl)propylamino)-3-methylbutanamideC23H34N2O2[α]D23=-7.4 (c 1.12, CHCl3).Source of chirality: (S)-valineAbsolute configuration: (S,R)
(S)-N,N-Diethyl-2-((R)-2-hydroxy-2-methyl-1-(naphthalen-2-yl)propylamino)-3-methylbutanamideC20H34N2O3[α]D22=+95.8 (c 1.10, CHCl3).Source of chirality: (S)-valineAbsolute configuration: (S,R)
(S)-N,N-Diethyl-2-((S)-2-hydroxy-2-methyl-1-(naphthalen-2-yl)propylamino)-3-methylbutanamideC20H34N2O3[α]D24=+57.6 (c 1.03, CHCl3).Source of chirality: (S)-valineAbsolute configuration: (S,S)
(S)-2-((R)-(1-Hydroxycyclopentyl)(phenyl)methylamino)-N,N,3-trimethylbutanamideC19H30N2O2[α]D24=-54.4 (c 1.04, CHCl3).Source of chirality: (S)-valineAbsolute configuration: (S,R)
(S)-2-((R)-(1-Hydroxycyclohexyl)(phenyl)methylamino)-N,N,3-trimethylbutanamideC20H32N2O2[α]D22=+35.1 (c 1.15, CHCl3).Source of chirality: (S)-valineAbsolute configuration: (S,S)
(S)-N,N-Diethyl-2-((S)-2-hydroxy-2-methyl-1-phenylpropylamino)-3-methylbutanamideC19H32N2O2[α]D22=+35.6 (c 1.26, CHCl3).Source of chirality: (S)-valineAbsolute configuration: (S,S)
(S)-2-((S)-2-Hydroxy-2-methyl-1-phenylpropylamino)-N,N,4-trimethylpentanamidC18H30N2O2[α]D24=+21.1 (c 0.90, CHCl3).Source of chirality: (S)-isoleucineAbsolute configuration: (S,S)
(S)-N,N-Diethyl-2-((S)-2-hydroxy-2-methyl-1-(naphthalen-1-yl)propylamino)-3-methylbutanamideC23H34N2O2[α]D22=-22.0 (c 0.95, CHCl3).Source of chirality: (S)-valineAbsolute configuration: (S,S)
Journal: Tetrahedron: Asymmetry - Volume 22, Issues 20–22, 30 November 2011, Pages 1906–1917