| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346243 | 980249 | 2011 | 9 صفحه PDF | دانلود رایگان |
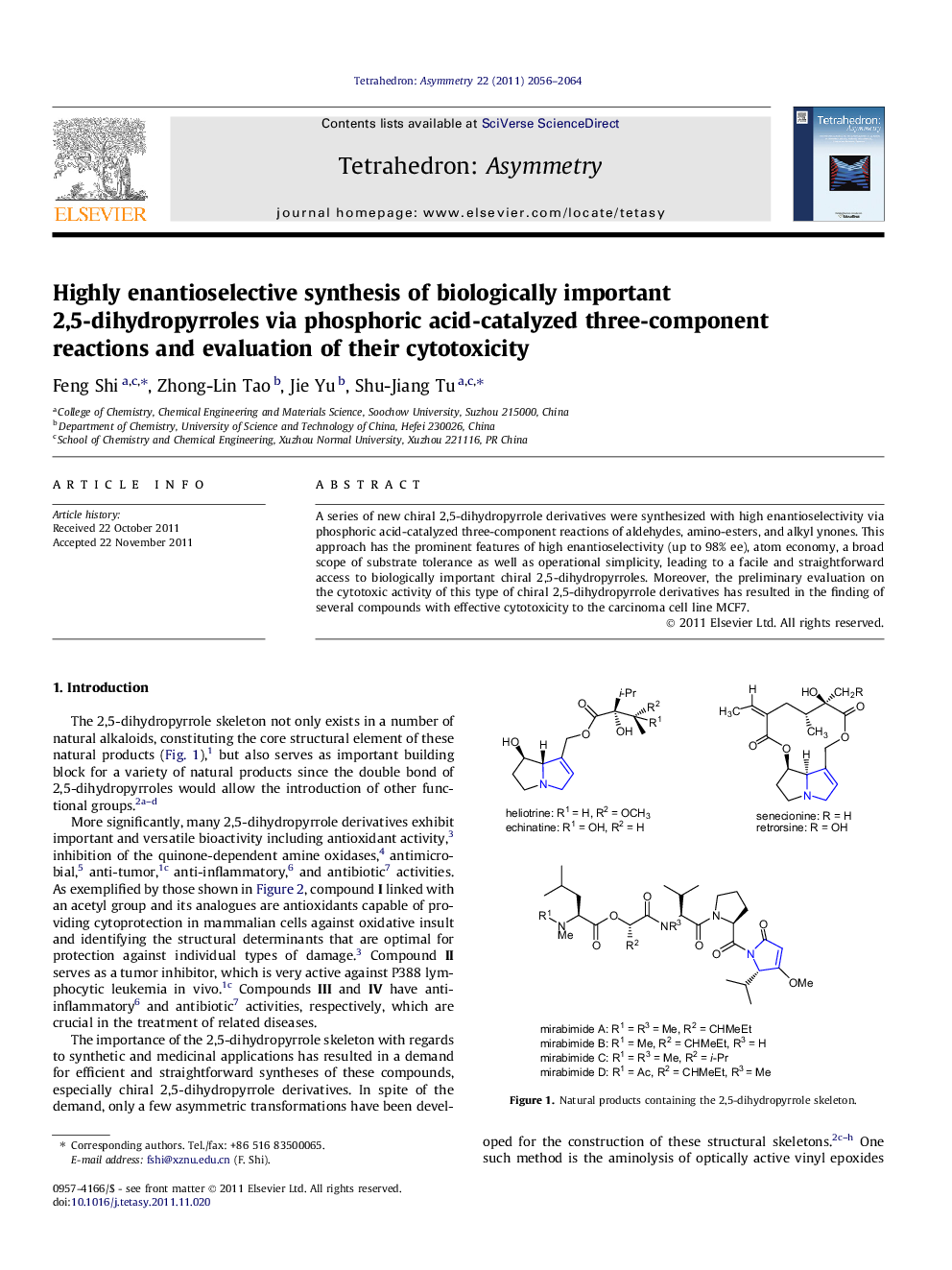
A series of new chiral 2,5-dihydropyrrole derivatives were synthesized with high enantioselectivity via phosphoric acid-catalyzed three-component reactions of aldehydes, amino-esters, and alkyl ynones. This approach has the prominent features of high enantioselectivity (up to 98% ee), atom economy, a broad scope of substrate tolerance as well as operational simplicity, leading to a facile and straightforward access to biologically important chiral 2,5-dihydropyrroles. Moreover, the preliminary evaluation on the cytotoxic activity of this type of chiral 2,5-dihydropyrrole derivatives has resulted in the finding of several compounds with effective cytotoxicity to the carcinoma cell line MCF7.
Figure optionsDownload as PowerPoint slide
(5S)-Diethyl 4-acetyl-5-(4-nitrophenyl)-1H-pyrrole-2,2(5H)-dicarboxylateC18H20N2O7[α]D20=+178.4 (c 0.5, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-(3-nitrophenyl)-1H-pyrrole-2,2(5H)-dicarboxylateC18H20N2O7[α]D20=+249.2 (c 0.3, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-(2-nitrophenyl)-1H-pyrrole-2,2(5H)-dicarboxylateC18H20N2O7[α]D20=+364.4 (c 0.4, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-(4-fluorophenyl)-1H-pyrrole-2,2(5H)-dicarboxylateC18H20FNO5[α]D20=+170.4 (c 0.2, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-(4-bromophenyl)-1H-pyrrole-2,2(5H)-dicarboxylateC18H20BrNO5[α]D20=+158.1 (c 0.5, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-(4-cyanophenyl)-1H-pyrrole-2,2(5H)-dicarboxylateC19H20N2O5[α]D20=+215.0 (c 0.3, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-(2-bromophenyl)-1H-pyrrole-2,2(5H)-dicarboxylateC18H20BrNO5[α]D20=+117.4 (c 0.2, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-(4-(methylsulfonyl)phenyl)-1H-pyrrole-2,2(5H)-dicarboxylateC19H23NO7S[α]D20=+181.4 (c 0.3, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-phenyl-1H-pyrrole-2,2(5H)-dicarboxylateC18H21NO5[α]D20=+122.2 (c 0.1, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-(naphthalen-2-yl)-1H-pyrrole-2,2(5H)-dicarboxylateC22H23NO5[α]D20=+150.0 (c 0.1, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-(3,4-dichlorophenyl)-1H-pyrrole-2,2(5H)-dicarboxylateC18H19Cl2NO5[α]D20=+126.1 (c 0.2, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-p-tolyl-1H-pyrrole-2,2(5H)-dicarboxylateC19H23NO5[α]D20=+135.1 (c 0.4, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-(4-methoxyphenyl)-1H-pyrrole-2,2(5H)-dicarboxylateC19H23NO6[α]D20=+147.8 (c 0.4, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-(3-methoxyphenyl)-1H-pyrrole-2,2(5H)-dicarboxylateC19H23NO6[α]D20=+189.8 (c 0.1, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-(thiophen-2-yl)-1H-pyrrole-2,2(5H)-dicarboxylateC16H19NO5S[α]D20=+192.3 (c 0.03, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-(furan-2-yl)-1H-pyrrole-2,2(5H)-dicarboxylateC16H19NO6[α]D20=+63.2 (c 0.2, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-(4-methoxystyryl)-1H-pyrrole-2,2(5H)-dicarboxylateC21H25NO6[α]D20=+15.3 (c 0.1, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-acetyl-5-cyclohexyl-1H-pyrrole-2,2(5H)-dicarboxylateC18H27NO5[α]D20=+105.0 (c 0.04, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 5-(4-nitrophenyl)-4-nonanoyl-1H-pyrrole-2,2(5H)-dicarboxylateC25H34N2O7[α]D20=+167.2 (c 0.634, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 5-(4-nitrophenyl)-4-(3-phenylpropanoyl)-1H-pyrrole-2,2(5H)-dicarboxylateC25H26N2O7[α]D20=+174.1 (c 0.692, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-(2-ethylbutanoyl)-5-(4-nitrophenyl)-1H-pyrrole-2,2(5H)-dicarboxylateC22H28N2O7[α]D20=+183.0 (c 0.436, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
(5S)-Diethyl 4-(cyclohexanecarbonyl)-5-(4-nitrophenyl)-1H-pyrrole-2,2(5H)-dicarboxylateC23H28N2O7[α]D20=+191.8 (c 0.61, CHCl3)Source of chirality: the catalystAbsolute configuration: (5S)
Journal: Tetrahedron: Asymmetry - Volume 22, Issue 23, 15 December 2011, Pages 2056–2064