| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346288 | 1500349 | 2013 | 10 صفحه PDF | دانلود رایگان |
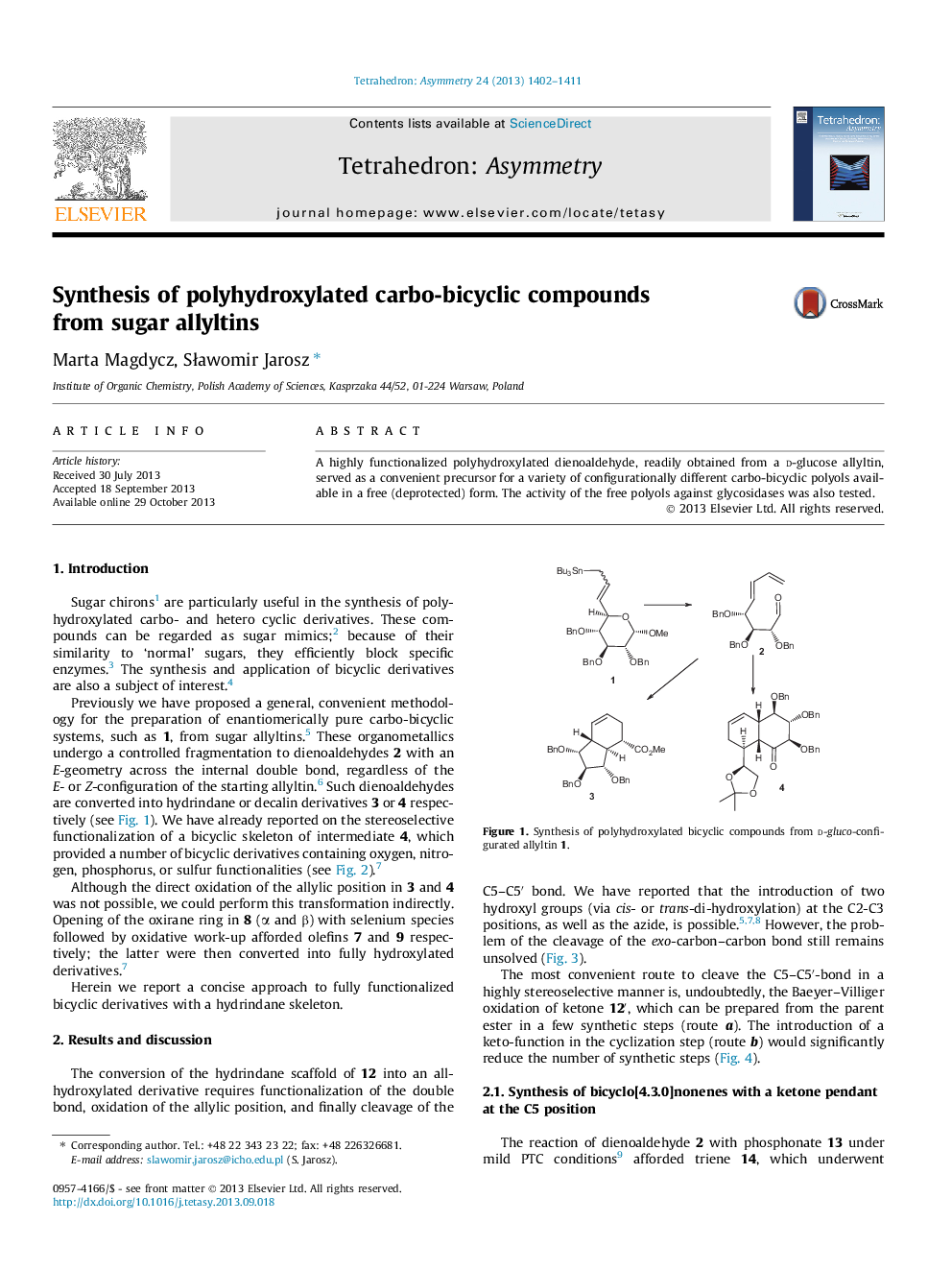
A highly functionalized polyhydroxylated dienoaldehyde, readily obtained from a d-glucose allyltin, served as a convenient precursor for a variety of configurationally different carbo-bicyclic polyols available in a free (deprotected) form. The activity of the free polyols against glycosidases was also tested.
Figure optionsDownload as PowerPoint slide
(1R,5S,6S,7S,8S,9R)-7,8,9-Tri-O-benzyl-5-methylcarbonyl-bicyclo[4,3,0]non-2-eneC32H34O4[α]Drt=+43.8 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1R,5S,6S,7S,8S,9R)
(1S,5R,6R,7S,8S,9R)-7,8,9-Tri-O-benzyl-5-methylcarbonyl-bicyclo[4,3,0]non-2-eneC32H34O4[α]Drt=+13.6 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1S,5R,6R,7S,8S,9R)
(1R,2S,3R,5S,6S,7S,8R,9R)-7,8,9-Tri-O-benzyl-5-methyl-carbonyl-2,3-dihydroxybicyclo[4.3.0]nonaneC32H36O6[α]Drt=-29.5 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1R,2S,3R,5S,6S,7S,8R,9R)
(1R,2R,3S,5S,6S,7S,8R,9R)-7,8,9-Tri-O-benzyl-5-methyl-carbonyl-2,3-dihydroxybicyclo[4.3.0]nonaneC32H36O6[α]Drt=-15.6 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1R,2R,3S,5S,6S,7S,8R,9R)
(1R,2R,3R,5S,6S,7S,8R,9R)-7,8,9-Tri-O-benzyl-5-methyl-carbonyl-2,3-dihydroxybicyclo[4.3.0]nonaneC32H36O6[α]Drt=-2.3 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1R,2R,3R,5S,6S,7S,8R,9R)
(1R,2S,3R,5S,6S,7S,8R,9R)-7,8,9-Tri-O-benzyl-5-O-acetyl-2,3-dihydroxy-bicyclo[4.3.0]nonaneC32H36O7[α]Drt=-46.9 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1R,2S,3R,5S,6S,7S,8R,9R)
(1R,2R,3S,5S,6S,7S,8R,9R)-7,8,9-Tri-O-benzyl-5-O-acetyl-2,3-dihydroxy-bicyclo[4.3.0]nonaneC32H36O7[α]Drt=-51.3 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1R,2R,3S,5S,6S,7S,8R,9R)
(1R,2R,3R,5S,6S,7S,8R,9R)-7,8,9-Tri-O-benzyl-5-O-acetyl-2,3-dihydroxy-bicyclo[4.3.0]nonaneC32H36O7[α]Drt=-32.3 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1R,2R,3R,5S,6S,7S,8R,9R)
(1S,2R,3R,5R,6R,7S,8S,9R)-2,3,7,8,9-Penta-O-benzyl-5-methylcarbonyl- bicyclo[4.3.0]nonaneC32H36O7[α]Drt=-2.3 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1S,2R,3R,5R,6R,7S,8S,9R)
(1S,2R,3R,5R,6R,7S,8S,9R)-2,3,7,8,9-Penta-O-benzyl-5-hydroxy-bicyclo [4.3.0]nonaneC46H48O6[α]Drt=-18.0 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1S,2R,3R,5R,6R,7S,8S,9R)
(1S,2R,3R,5R,6R,7S,8S,9R)-2,3,7,8,9-Penta-O-benzylo-5-O-mesyl-bicyclo [4.3.0]nonaneC45H48O8S[α]Drt=-7.4 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1S,2R,3R,5R,6R,7S,8S,9R)
(1S,2R,3R,5R,6R,7S,8S,9R)-2,3,7,8,9-penta-O-benzyl-5-O-acetyl-bicyclo[4.3.0]nonaneC46H48O7[α]Drt=-36.5 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1S,2R,3R,5R,6R,7S,8S,9R)
(1S,2R,3S,5R,6R,7S,8S,9R)-7,8,9-Tri-O-benzyl-5-methylocarbonyl-2,3-epoxy bicyclo[4,3,0]nonaneC32H34O5[α]Drt=-12.5 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1S,2R,3S,5R,6R,7S,8S,9R)
(1S,2S,3S,5R,6R,7S,8R,9R)-7,8,9-Tri-O-benzyl-5-methylcarbonyl-2,3-dihydroxybicyclo[4.3.0]nonaneC32H36O6[α]Drt=+32.9 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1S,2S,3S,5R,6R,7S,8R,9R)
(1S,2R,3S,5R,6R,7S,8R,9R)-7,8,9-Tri-O-benzyl-5-methyl-carbonyl-2,3-dihydroxybicyclo[4.3.0]nonaneC32H36O6[α]Drt=+14.8 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1S,2R,3S,5R,6R,7S,8R,9R)
(1S,2S,3S,5R,6R,7S,8R,9R)-7,8,9-Tri-O-benzyl-5-O-acetyl-2,3-dihydroxy-bicyclo[4.3.0]nonaneC32H36O7[α]Drt=+23.2 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1S,2S,3S,5R,6R,7S,8R,9R)
(1S,2R,3S,5R,6R,7S,8R,9R)-2,3,7,8,9-Penta-O-benzyl-5-O-acetyl-bicyclo [4.3.0]nonaneC46H48O7[α]Drt=+46.5 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1S,2R,3S,5R,6R,7S,8R,9R)
(1S,2R,3S,5R,6R,7S,8R,9R)-2,3,7,8,9-Penta-O-benzyl-5-O-mesyl-bicyclo[4.3.0] nonaneC45H48O8S[α]Drt=+47.8 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1S,2R,3S,5R,6R,7S,8R,9R)
(1S,2R,3S,5S,6R,7S,8R,9R)-2,3,7,8,9-Penta-O-benzyl-5-hydroxy-bicyclo[4.3.0] nonaneC44H46O6[α]Drt=+45.6 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1S,2R,3S,5S,6R,7S,8R,9R)
(1S,2R,3S,5S,6R,7S,8R,9R)-2,3,7,8,9-Penta-O-benzyl-5-O-acetyl-bicyclo[4.3.0] nonaneC46H48O7[α]Drt=+7.8 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1S,2R,3S,5S,6R,7S,8R,9R)
(1R,2S,3R,5S,6S,7S,8R,9R)-2,3,5,7,8,9-Hexahydroxybicyclo[4.3.0]nonaneC9H16O6[α]Drt=-51.8 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1R,2S,3R,5S,6S,7S,8R,9R)
(1R,2S,3R,5S,6S,7S,8R,9R)-7,8,9-Tri-O-benzyl-2,3,5-trihydroxy-bicyclo[4.3.0] nonaneC30H34O6[α]Drt=-30.7 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1R,2S,3R,5S,6S,7S,8R,9R)
(1R,2R,3S,5S,6S,7S,8R,9R)-2,3,5,7,8,9-Hexahydroxybicyclo[4.3.0]nonaneC9H16O6[α]Drt=-34.3 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1R,2R,3S,5S,6S,7S,8R,9R)
(1R),2R,3S,5S,6S,7S,8R,9R)-7,8,9-Tri-O-benzyl-2,3,5-trihydroxy-bicyclo[4.3.0] nonaneC30H34O6[α]Drt=-33.1 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1R,2R,3S,5S,6S,7S,8R,9R)
(1R,2R,3R,5S,6S,7S,8R,9R)-2,3,5,7,8,9-Hexahydroxybicyclo[4.3.0]nonaneC9H16O6[α]Drt=-2.4 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1R,2R,3R,5S,6S,7S,8R,9R)
(1R,2R,3R,5S,6S,7S,8R,9R)-7,8,9-Tri-O-benzyl-2,3,5-trihydroxy-bicyclo [4.3.0]nonaneC30H34O6[α]Drt=-13.1 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1R,2R,3R,5S,6S,7S,8R,9R)
(1S,2S,3S,5R,6R,7S,8R,9R)-2,3,5,7,8,9-Hexahydroxybicyclo[4.3.0]nonaneC9H16O6[α]Drt=+14.8 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienalAbsolute configuration: (1S,2S,3S,5R,6R,7S,8R,9R)
(1S,2S,3S,5R,6R,7S,8R,9R)-7,8,9-Tri-O-benzyl-2,3,5-trihydroxy-bicyclo[4.3.0] nonaneC30H34O6[α]Drt=+19.9 (c 1.0, CHCl3)Source of chirality: (E)-(2R,3S,4R)-2,3,4-Tris-benzyloxy-octa-5,7-dienallAbsolute configuration: (1S,2S,3S,5R,6R,7S,8R,9R)
Journal: Tetrahedron: Asymmetry - Volume 24, Issues 21–22, 30 November 2013, Pages 1402–1411