| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346290 | 1500349 | 2013 | 4 صفحه PDF | دانلود رایگان |
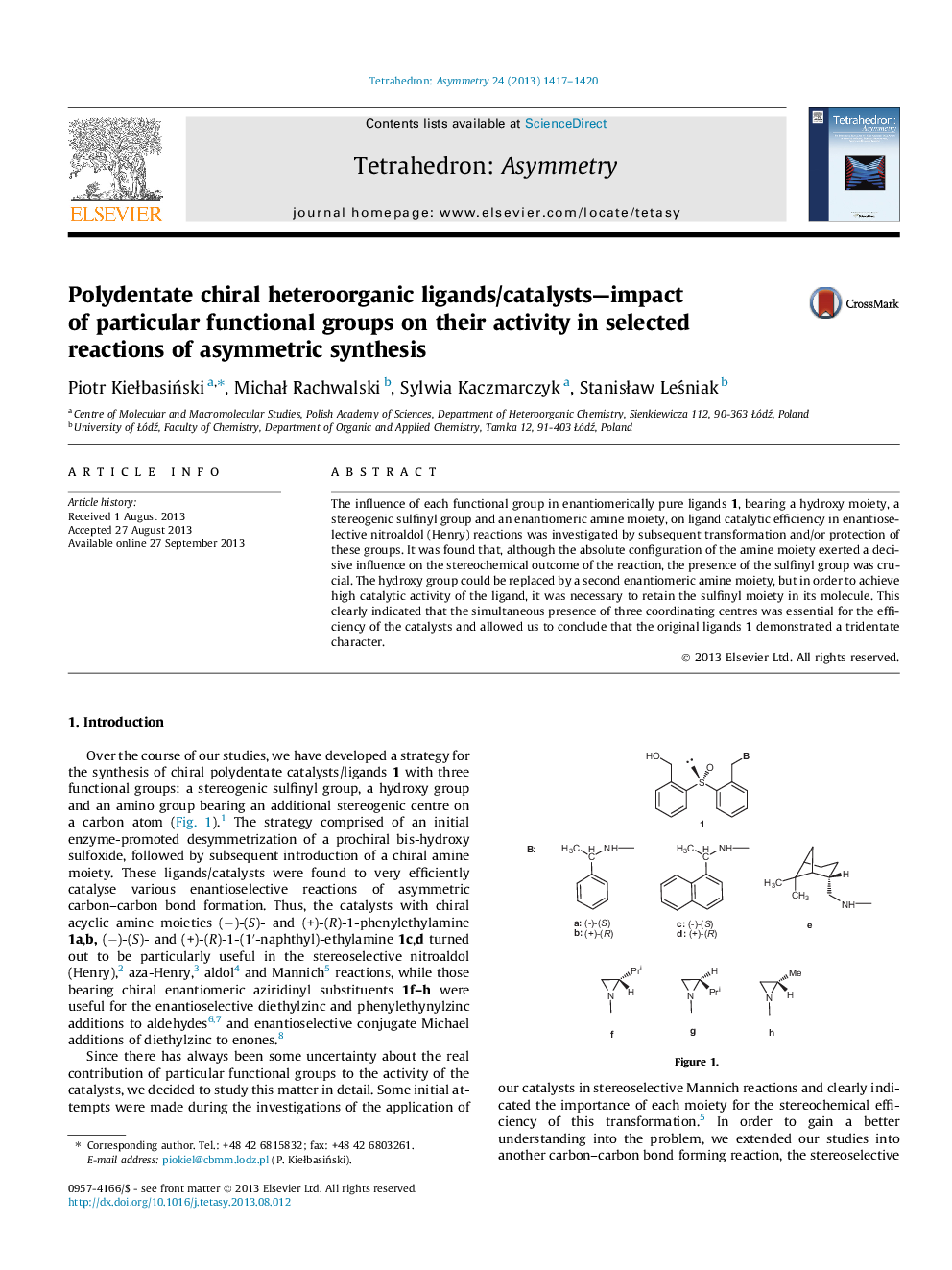
The influence of each functional group in enantiomerically pure ligands 1, bearing a hydroxy moiety, a stereogenic sulfinyl group and an enantiomeric amine moiety, on ligand catalytic efficiency in enantioselective nitroaldol (Henry) reactions was investigated by subsequent transformation and/or protection of these groups. It was found that, although the absolute configuration of the amine moiety exerted a decisive influence on the stereochemical outcome of the reaction, the presence of the sulfinyl group was crucial. The hydroxy group could be replaced by a second enantiomeric amine moiety, but in order to achieve high catalytic activity of the ligand, it was necessary to retain the sulfinyl moiety in its molecule. This clearly indicated that the simultaneous presence of three coordinating centres was essential for the efficiency of the catalysts and allowed us to conclude that the original ligands 1 demonstrated a tridentate character.
Figure optionsDownload as PowerPoint slide
1-(2′-Chlorophenyl)-2-nitroethanolC8H8ClNO3Ee = 92%[α]Drt=-53.4 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (R) (literature data)
1-(2′-Methoxyphenyl)-2-nitroethanolC9H11NO4Ee = 97%[α]Drt=-46.0 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (R) (literature data)
1-Phenyl-2-nitroethanolC8H9NO3Ee = 98%[α]Drt=-22.0 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (R) (literature data)
1-(2′-Nitrophenyl)-2-nitroethanolC8H8N2O5Ee = 93%[α]Drt=+235.7 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (R) (literature data)
1-Nitro-4-phenylbutan-2-olC10H13NO3Ee = 90%[α]Drt=+15.0 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (R) (literature data)
1-Nitrohexan-2-olC6H13NO3Ee = 94%[α]Drt=-9.3 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (R) (literature data)
Journal: Tetrahedron: Asymmetry - Volume 24, Issues 21–22, 30 November 2013, Pages 1417–1420