| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346339 | 980254 | 2011 | 7 صفحه PDF | دانلود رایگان |
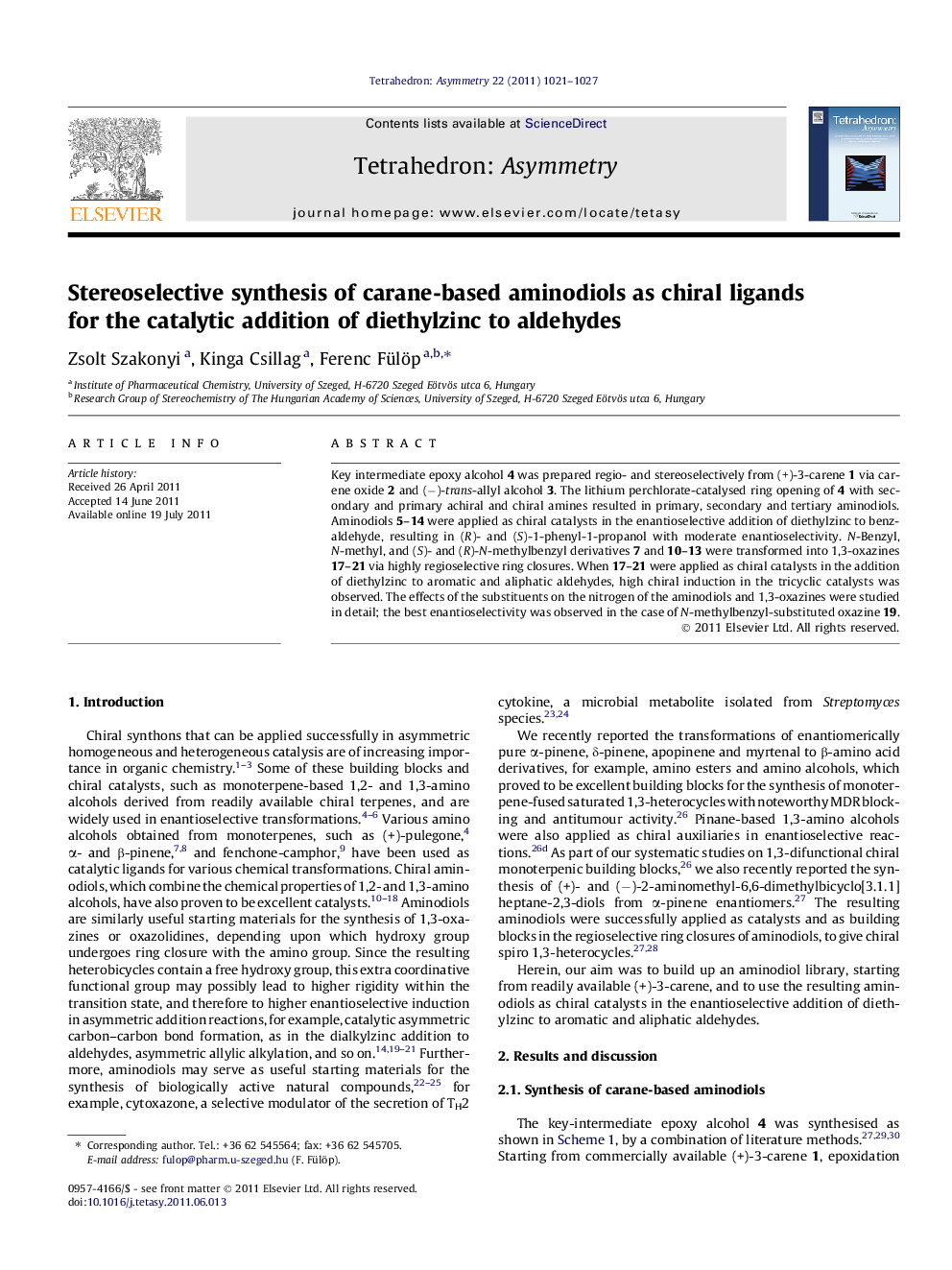
Key intermediate epoxy alcohol 4 was prepared regio- and stereoselectively from (+)-3-carene 1 via carene oxide 2 and (−)-trans-allyl alcohol 3. The lithium perchlorate-catalysed ring opening of 4 with secondary and primary achiral and chiral amines resulted in primary, secondary and tertiary aminodiols. Aminodiols 5–14 were applied as chiral catalysts in the enantioselective addition of diethylzinc to benzaldehyde, resulting in (R)- and (S)-1-phenyl-1-propanol with moderate enantioselectivity. N-Benzyl, N-methyl, and (S)- and (R)-N-methylbenzyl derivatives 7 and 10–13 were transformed into 1,3-oxazines 17–21 via highly regioselective ring closures. When 17–21 were applied as chiral catalysts in the addition of diethylzinc to aromatic and aliphatic aldehydes, high chiral induction in the tricyclic catalysts was observed. The effects of the substituents on the nitrogen of the aminodiols and 1,3-oxazines were studied in detail; the best enantioselectivity was observed in the case of N-methylbenzyl-substituted oxazine 19.
Figure optionsDownload as PowerPoint slide
(1S,2′R,4R,6R)-7,7-Dimethylspiro[bicyclo[4.1.0]heptane-3,2′-oxiran]-4-olC10H16O2[α]D20=-48.0 (c 0.25, MeOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1S,2′R,4R,6R)
(1S,3R,4R,6R)-3-(Benzyl(methyl)amino)methyl-7,7-dimethylbicyclo[4.1.0]heptane-3,4-diolC18H27NO2[α]D20=+35.0 (c 0.125, EtOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1S,3R,4R,6R)
(1S,3R,4R,6R)-7,7-Dimethyl-3-((methylamino)methyl)bicyclo[4.1.0]heptane-3,4-diolC11H21NO2[α]D20=+24.0 (c 0.25, MeOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1S,3R,4R,6R)
(1S,3R,4R,6R)-3-(Dibenzylamino)methyl-7,7-dimethylbicyclo[4.1.0]heptane-3,4-diolC24H31NO2[α]D20=+31.0 (c 0.125, EtOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1S,3R,4R,6R)
(1S,3R,4R,6R)-3-(Benzylamino)methyl-7,7-dimethylbicyclo[4.1.0]heptane-3,4-diolC17H25NO2[α]D20=+23.0 (c 0.125, EtOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1S,3R,4R,6R)
(1S,3R,4R,6R)-3-(Benzyl((R)-1′-phenylethyl)amino)methyl-7,7-dimethylbicyclo[4.1.0]heptane-3,4-diolC25H33NO2[α]D20=+56.0 (c 0.125, EtOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1S,3R,4R,6R, 1′R)
(1S,3R,4R,6R)-3-(Benzyl((S)-1′-phenylethyl)amino)methyl-7,7-dimethylbicyclo[4.1.0]heptane-3,4-diolC25H33NO2[α]D20=-8.0 (c 0.125, EtOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1S,3R,4R,6R,1′S)
(1S,3R,4R,6R)-3-Aminomethyl-7,7-dimethylbicyclo[4.1.0]heptane-3,4-diolC10H19NO2[α]D20=-32.0 (c 0.125, EtOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1S,3R,4R,6R)
(1S,3R,4R,6R)-7,7-Dimethyl-3-(((R)-1-phenylethylamino)methyl)bicyclo[4.1.0]heptane-3,4-diolC18H27NO2[α]D20=+65.0 (c 0.125, EtOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1S,3R,4R,6R, 1′R)
(1S,3R,4R,6R)-7,7-Dimethyl-3-(((S)-1-phenylethylamino)methyl)bicyclo[4.1.0]heptane-3,4-diolC18H27NO2[α]D20=-4.0 (c 0.125, EtOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1S,3R,4R,6R,1′S)
(1S,3R,4R,6R)-3-(Isopropylamino)methyl-7,7-dimethylbicyclo[4.1.0]heptane-3,4-diolC13H25NO2[α]D20=+44.0 (c 0.125, EtOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1S,3R,4R,6R)
(1aR,2aR,6aR,7aS)-5-Benzyl-1,1-dimethyl-6a-hydroxydecahydro-3-oxa-5-azacyclopropa[g]naphthaleneC18H25NO2[α]D20=-61.0 (c 0.125, EtOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1aR,2aR,6aR,7aS)
(1aR,2aR,6aR,7aS)-6a-Hydroxy-1,1,5-trimethyldecahydro-3-oxa-5-azacyclopropa[g]naphthaleneC12H21NO2[α]D20=-13.0 (c 0.125, EtOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1aR,2aR,6aR,7aS)
(1aR,2aR,6aR,7aS)-6a-hydroxy-1,1-dimethyl-5-((R)-1-phenylethyl)decahydro-3-oxa-5-azacyclopropa[g]naphthaleneC19H27NO2[α]D20=-18.0 (c 0.125, EtOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1aR,2aR,6aR,7aS,1′R)
(1aR,2aR,6aR,7aS)-6a-Hydroxy-1,1-dimethyl-5-((S)-1-phenylethyl)decahydro-3-oxa-5-azacyclopropa[g]naphthaleneC19H27NO2[α]D20=-44.0 (c 0.125, EtOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: (1aR,2aR,6aR,7aS,1′S)
(1aR,2aR,6aR,7aS)-1,1-Dimethyl-6a-hydroxy-5-isopropyldecahydro-3-oxa-5-azacyclopropa[g]naphthaleneC14H25NO2[α]D20=-22.0 (c 0.125, MeOH)Source of chirality: (1S,6R)-3-careneAbsolute configuration: 1(aR,2aR,6aR,7aS)
Journal: Tetrahedron: Asymmetry - Volume 22, Issue 9, 15 May 2011, Pages 1021–1027