| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346427 | 1500330 | 2016 | 8 صفحه PDF | دانلود رایگان |
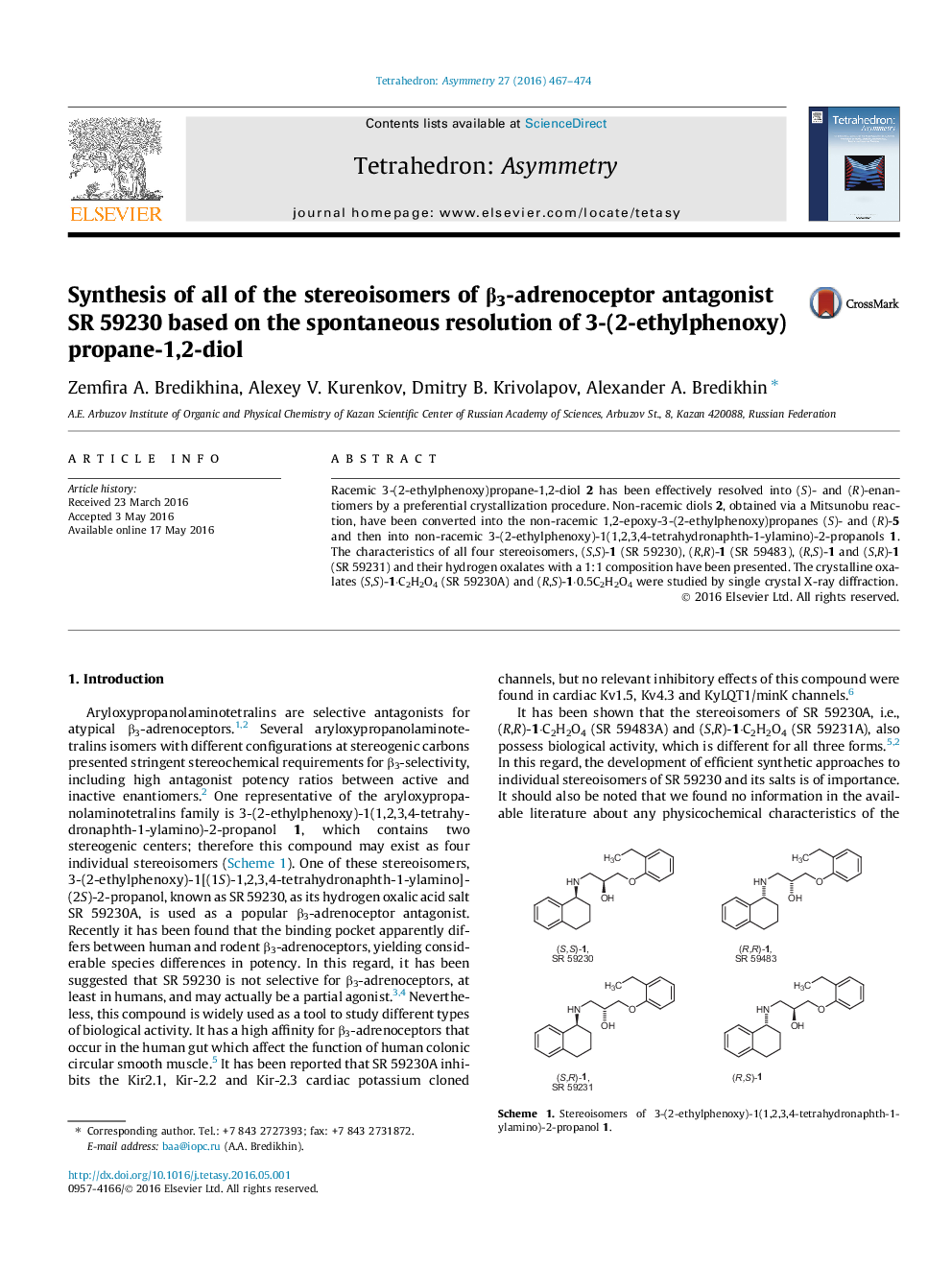
Racemic 3-(2-ethylphenoxy)propane-1,2-diol 2 has been effectively resolved into (S)- and (R)-enantiomers by a preferential crystallization procedure. Non-racemic diols 2, obtained via a Mitsunobu reaction, have been converted into the non-racemic 1,2-epoxy-3-(2-ethylphenoxy)propanes (S)- and (R)-5 and then into non-racemic 3-(2-ethylphenoxy)-1(1,2,3,4-tetrahydronaphth-1-ylamino)-2-propanols 1. The characteristics of all four stereoisomers, (S,S)-1 (SR 59230), (R,R)-1 (SR 59483), (R,S)-1 and (S,R)-1 (SR 59231) and their hydrogen oxalates with a 1:1 composition have been presented. The crystalline oxalates (S,S)-1·C2H2O4 (SR 59230A) and (R,S)-1·0.5C2H2O4 were studied by single crystal X-ray diffraction.
Figure optionsDownload as PowerPoint slide
(R)-3-(2-Ethylphenoxy)propane-1,2-diolC11H16O3Ee = 99.8% (ee from HPLC)[α]D20 = +13.0 (c 1, MTBE)Initial source of chirality: Resolution of racemic 3-(2-ethylphenoxy)propane-1,2-diol by preferential crystallizationAbsolute configuration: (R)
(R)-1,2-Epoxy-3-(2-ethylphenoxy)propaneC11H14O2Ee = 95.4% (ee from HPLC)[α]D20 = −14.8 (c 1.3, EtOH), [α]36520 = −35.8 (c 1.3, EtOH)Initial source of chirality: (R)-3-(2-ethylphenoxy)propane-1,2-diolAbsolute configuration: (R)
3-(2-Ethylphenoxy)-1[(1R)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2R)-2-propanol hydrogen oxalateC11H27NO2·C2H2O4Ee = 98.6% (ee from HPLC)[α]D20 = +18.3 (c 1.1, MeOH), [α]36520 = +70.6 (c 1.1, MeOH)Initial source of chirality: (3-(2-Ethylphenoxy)-1[(1R)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2R)-2-propanolAbsolute configuration: (R,R)
3-(2-Ethylphenoxy)-1[(1R)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2R)-2-propanolC11H27NO2Ee = 95.8% (ee from HPLC)[α]D20 = +14.0 (c 1.1, CHCl3), [α]36520 = +66.2 (c 1.1, CHCl3)[α]D20 = +14.0 (c 1.3, EtOH), [α]36520 = +66.8 (c 1.3, EtOH)Initial source of chirality: (R)-(−)-1,2,3,4-tetrahydro-1-naphthylamine and (R)-1,2-epoxy-3-(2-ethylphenoxy)propaneAbsolute configuration: (R,R)
3-(2-Ethylphenoxy)-1[(1R)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2S)-2-propanol hydrogen oxalateC11H27NO2·C2H2O4Ee = 99.1% (ee from HPLC)[α]D20 = −21.2 (c 1.0, DMSO), [α]36520 = −53.2 (c 1.0, DMSO)Initial source of chirality: (3-(2-Ethylphenoxy)-1[(1R)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2S)-2-propanolAbsolute configuration: (R,S)
3-(2-Ethylphenoxy)-1[(1R)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2S)-2-propanolC11H27NO2Ee = 98.1% (ee from HPLC)[α]D20 = −15.6 (c 1.1, CHCl3), [α]36520 = −32.4 (c 1.1, CHCl3)Initial source of chirality: (R)-(−)-1,2,3,4-tetrahydro-1-naphthylamine and (S)-1,2-epoxy-3-(2-ethylphenoxy)propaneAbsolute configuration: (R,S)
Journal: Tetrahedron: Asymmetry - Volume 27, Issues 11–12, 1 July 2016, Pages 467–474