| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346448 | 980262 | 2011 | 6 صفحه PDF | دانلود رایگان |
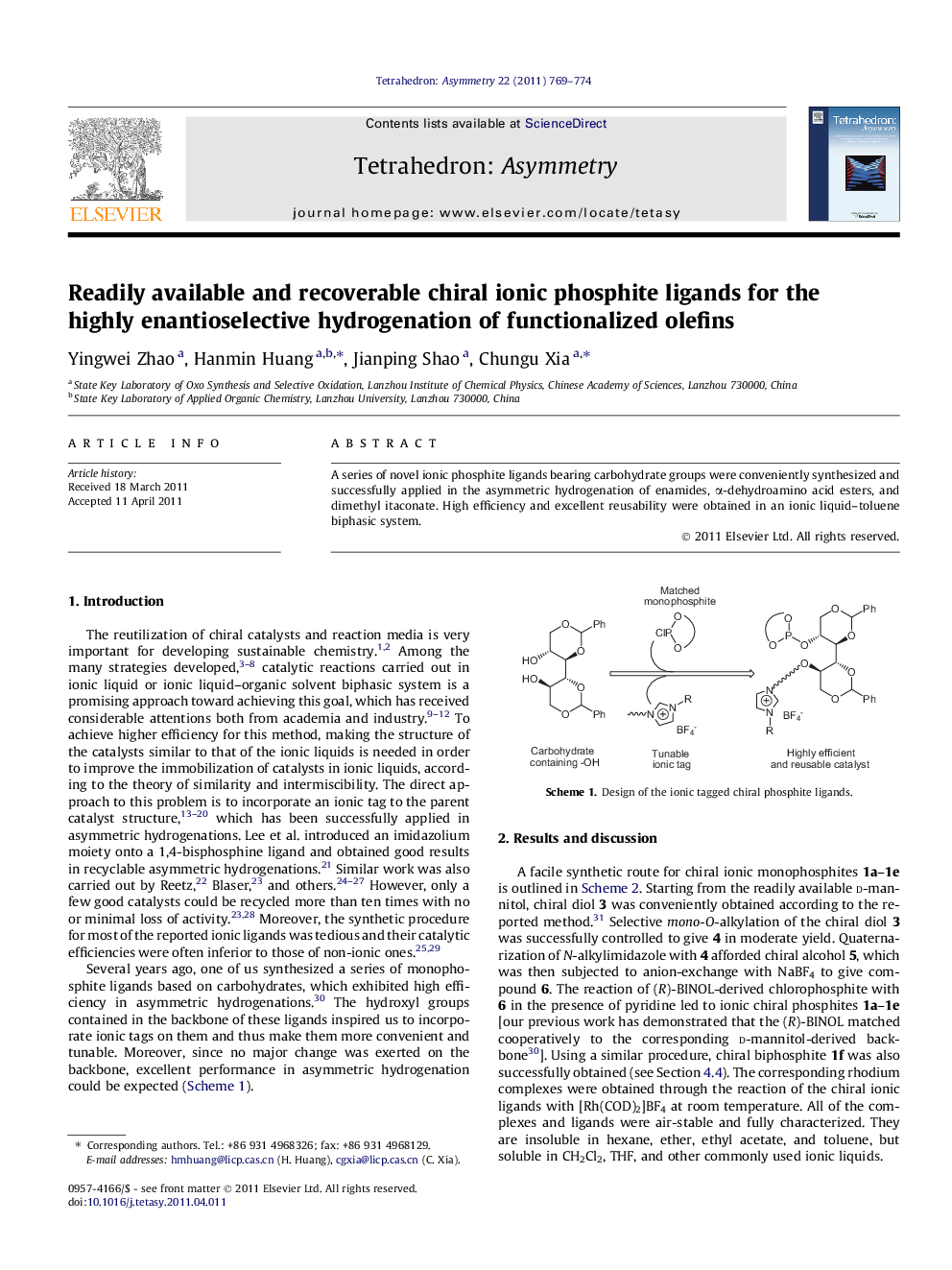
A series of novel ionic phosphite ligands bearing carbohydrate groups were conveniently synthesized and successfully applied in the asymmetric hydrogenation of enamides, α-dehydroamino acid esters, and dimethyl itaconate. High efficiency and excellent reusability were obtained in an ionic liquid–toluene biphasic system.
Figure optionsDownload as PowerPoint slide
1-(4-((4R,4′S,5R,5′R)-5′-(dinaphtho[2,1-d:1′,2′-f][1,3,2]dioxaphosphepin-4-yloxy)-2,2′-diphenyl-4,4′-bi(1,3-dioxan)-5-yloxy)butyl)-3-methyl-1H-imidazol-3-ium tetrafluoroborateC48H46BF4N2O8P[α]D25=-224.4 (c 0.45, CH2Cl2)Source of chirality: d-mannitol and (R)-BINOLAbsolute configuration: (4R,4′S,5R,5′R)
1-(4-((4R,4′S,5R,5′R)-5′-(Dinaphtho[2,1-d:1′,2′-f][1,3,2]dioxaphosphepin-4-yloxy)-2,2′-diphenyl-4,4′-bi(1,3-dioxan)-5-yloxy)butyl)-2,3-dimethyl-1H-imidazol-3-ium tetrafluoroborateC49H48BF4N2O8P[α]D25=-225.0 (c 0.52, CH2Cl2)Source of chirality: d-mannitol and (R)-BINOLAbsolute configuration: (4R,4′S,5R,5′R)
3-Butyl-1-(4-((4R,4′S,5R,5′R)-5′-(dinaphtho[2,1-d:1′,2′-f][1,3,2]dioxaphosphepin-4-yloxy)-2,2′-diphenyl-4,4′-bi(1,3-dioxan)-5-yloxy)butyl)-1H-imidazol-3-ium tetrafluoroborateC51H52BF4N2O8P[α]D25=-213.3 (c 0.48, CH2Cl2)Source of chirality: d-mannitol and (R)-BINOLAbsolute configuration: (4R,4′S,5R,5′R)
1-(6-((4R,4′S,5R,5′R)-5′-(Dinaphtho[2,1-d:1′,2′-f][1,3,2]dioxaphosphepin-4-yloxy)-2,2′-diphenyl-4,4′-bi(1,3-dioxan)-5-yloxy)hexyl)-3-methyl-1H-imidazol-3-ium tetrafluoroborateC50H50BF4N2O8P[α]D25=-216.0 (c 0.42, CH2Cl2)Source of chirality: d-mannitol and (R)-BINOLAbsolute configuration: (4R,4′S,5R,5′R)
1-(12-((4R,4′S,5R,5′R)-5′-(Dinaphtho[2,1-d:1′,2′-f][1,3,2]dioxaphosphepin-4-yloxy)-2,2′-diphenyl-4,4′-bi(1,3-dioxan)-5-yloxy)dodecyl)-3-methyl-1H-imidazol-3-iumC56H62BF4N2O8P[α]D25=-194.4 (c 0.43, CH2Cl2)Source of chirality: d-mannitol and (R)-BINOLAbsolute configuration: (4R,4′S,5R,5′R)
1,3-Bis(4-((4R,4′S,5R,5′R)-5′-((11bR)-dinaphtho[2,1-d:1′,2′-f][1,3,2]dioxaphosphepin-4-yloxy)-2,2′-diphenyl-4,4′-bi(1,3-dioxan)-5-yloxy)butyl)-1H-imidazol-3-ium tetrafluoroborateC91H83 BF4N2O16P2[α]D25=-230.7 (c 0.44, CH2Cl2)Source of chirality: d-mannitol and (R)-BINOLAbsolute configuration: (4R,4′S,5R,5′R) (11bR)
Journal: Tetrahedron: Asymmetry - Volume 22, Issue 7, 11 April 2011, Pages 769–774