| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346449 | 980262 | 2011 | 5 صفحه PDF | دانلود رایگان |
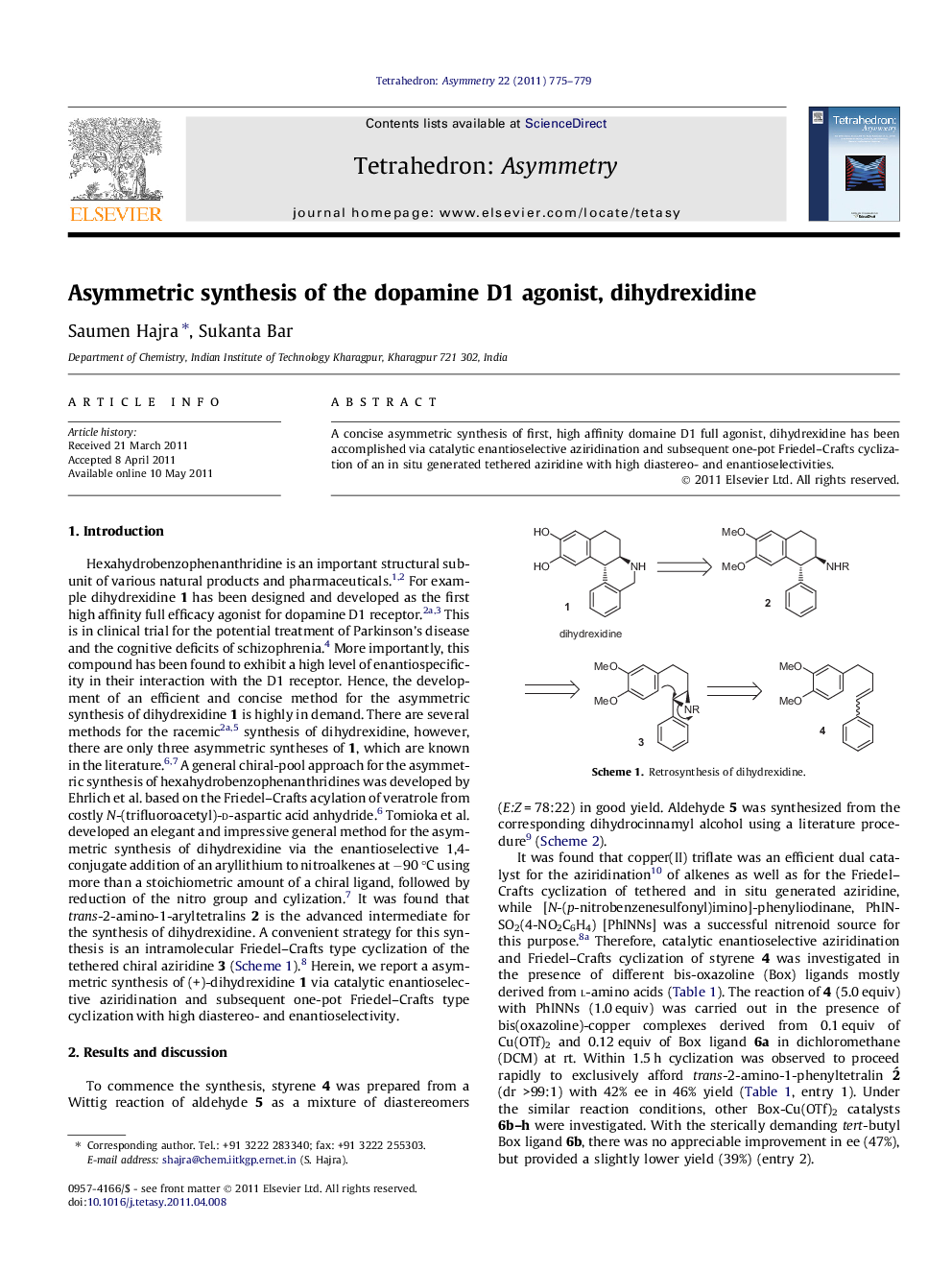
A concise asymmetric synthesis of first, high affinity domaine D1 full agonist, dihydrexidine has been accomplished via catalytic enantioselective aziridination and subsequent one-pot Friedel–Crafts cyclization of an in situ generated tethered aziridine with high diastereo- and enantioselectivities.
Figure optionsDownload as PowerPoint slide
(1S,2R)-N-(6,7-Dimethoxy-1-phenyl-1,2,3,4-tetrahydro-naphthalen-2-yl)-4-nitro-benzenesulfonamideC24H24N2O6SEe ⩾ 99%[α]D28=-52.0 (c 1.00, CHCl3)Source of chirality: (R)-bisoxazoline ligand 6e′Absolute configuration: (1S,2R)
(1S,2R)-N-(6,7-Dimethoxy-1-phenyl-1,2,3,4-tetrahydro-naphthalen-2-yl)-N-methoxymethyl-4-nitro-benzenesulfonamideC26H28N2O7SEe ⩾ 99%[α]D28=-36.0 (c 1.00, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (1S,2R)
(6aR,12bS)-10,11-Dimethoxy-6-(4-nitrobenzenesulfonyl)-5,6,6a,7,8,12b-hexahydrobenzo[a]phenanthridineC25H24N2O6SEe ⩾ 99%[α]D28=+30.2 (c 1.00, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (6aR,12bS)
(6aR,12bS)-10,11-Dimethoxy-5,6,6a,7,8,12b-hexahydrobenzo[a]phenanthridineC19H21NO2Ee ⩾ 99%[α]D28=-217.9 (c 1.1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (6aR,12bS)
(6aR,12bS)-10,11-Dihydroxy-5,6,6a,7,8,12b-hexahydrobenzo[a]phenanthridine hydrobromideC17H18BrNO2Ee ⩾ 99%[α]D28=37.0 (c 0.20, EtOH)Source of chirality: asymmetric synthesisAbsolute configuration: (6aR,12bS)
(6aR,12bS)-10,11-Dihydroxy-5,6,6a,7,8,12b-hexahydrobenzo[a]phenanthridine hydrochlorideC17H18ClNO2Ee ⩾ 99%[α]D28=79.9 (c 0.25, EtOH)Source of chirality: asymmetric synthesisAbsolute configuration: (6aR,12bS)
Journal: Tetrahedron: Asymmetry - Volume 22, Issue 7, 11 April 2011, Pages 775–779