| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346538 | 980266 | 2016 | 7 صفحه PDF | دانلود رایگان |
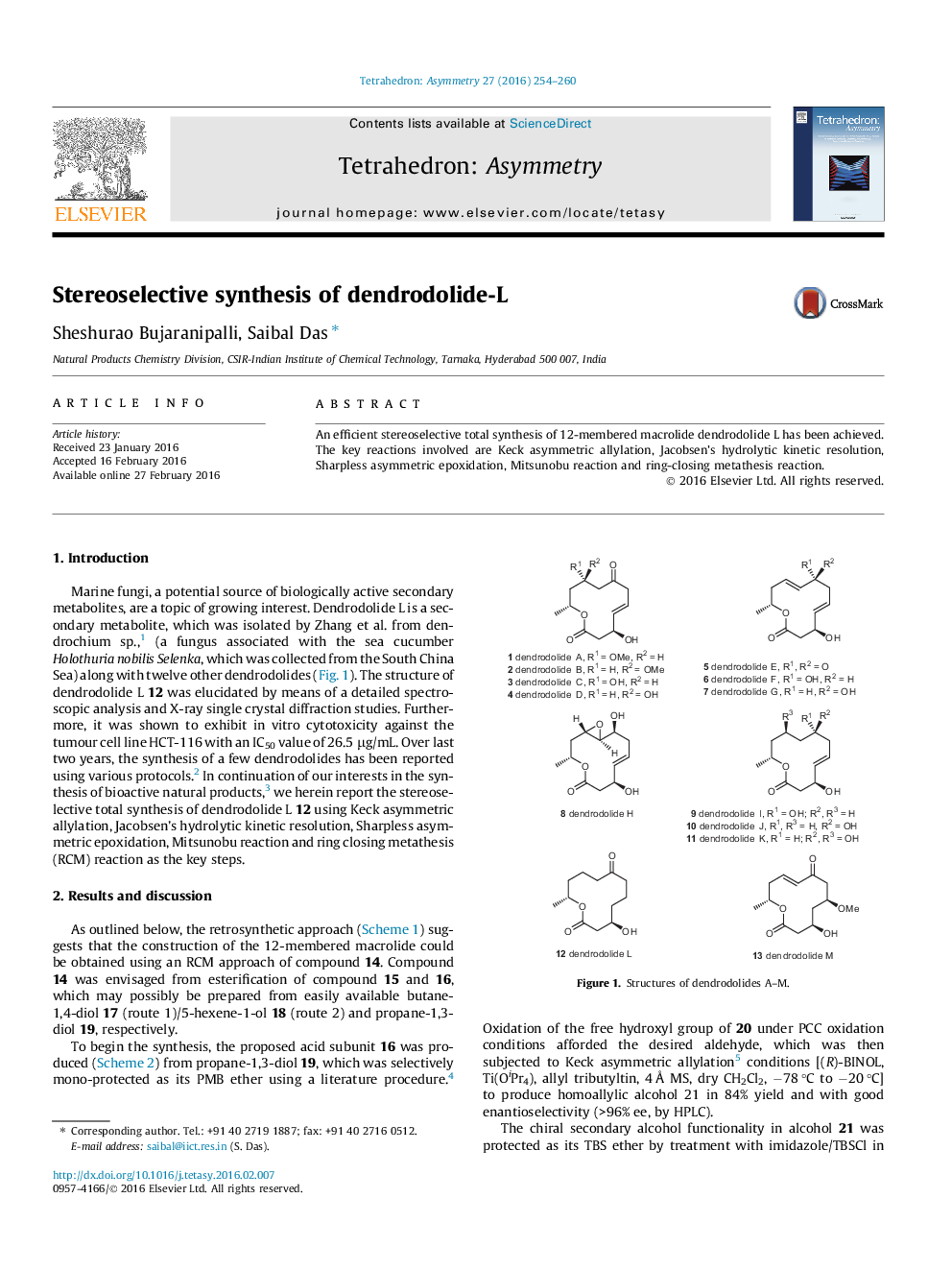
An efficient stereoselective total synthesis of 12-membered macrolide dendrodolide L has been achieved. The key reactions involved are Keck asymmetric allylation, Jacobsen’s hydrolytic kinetic resolution, Sharpless asymmetric epoxidation, Mitsunobu reaction and ring-closing metathesis reaction.
Figure optionsDownload as PowerPoint slide
((2S,3S)-3-(3-(Benzyloxy)propyl)oxiran-2-yl)methanolC13H18O3[α]D27 = −27.0 (c 1.8, CHCl3)Absolute configuration: (2S,3S)Source of chirality: Sharpless asymmetric epoxidation
((2S,3S)-3-(3-(Benzyloxy)propyl)oxiran-2-yl)methyl 4-methylbenzenesulfonateC20H24O5S[α]D27 = −23.8 (c 1.3, CHCl3)Absolute configuration: (2S,3S)Source of chirality: Sharpless asymmetric epoxidation
(S)-6-(Benzyloxy)hexan-2-olC13H20O2[α]D27 = +7.9 (c 2.0, CHCl3)Absolute configuration: (2S)Source of chirality: asymmetric synthesis
(R)-6-(Benzyloxy)-2-hydroxyhexyl 4-methylbenzenesulfonateC20H26O5S[α]D27 = +9.5 (c 0.64, CHCl3)Absolute configuration: (2R)Source of chirality: asymmetric synthesis
(S)-2-Hydroxy-2-((R)-tetrahydrofuran-2-yl)ethyl 4-methylbenzenesulfonateC13H18O5S[α]D27 = −12.1 (c 1.4, CHCl3)Absolute configuration: (2S,3R)Source of chirality: asymmetric synthesis
(R)-1-((4-Methoxybenzyl)oxy)hex-5-en-3-olC14H20O3[α]D27 = +3.6 (c 1.6, CHCl3)Absolute configuration: : (3R)Source of chirality: Keck asymmetric allylation
(R)-tert-Butyl((1-((4-methoxybenzyl)oxy)hex-5-en-3-yl)oxy)dimethylsilaneC20H34O3Si[α]D27 = −17.4 (c 1.9, CHCl3)Absolute configuration: (3R)Source of chirality: Keck asymmetric allylation
(R)-tert-Butyl((1-((4-methoxybenzyl)oxy)hex-5-en-3-yl)oxy)dimethylsilaneC12H26O2Si[α]D27 = −28.8 (c 1.24, CHCl3)Absolute configuration: (3R)Source of chirality: Keck asymmetric allylation
(R)-3-((tert-Butyldimethylsilyl)oxy)hex-5-enoic acidC12H24O3Si[α]D27 = −23.4 (c 2.0, CHCl3)Absolute configuration: (3R)Source of chirality: Keck asymmetric allylation
(7S)-7-((tert-Butyldimethylsilyl)oxy)oct-1-en-3-olC14H30O2Si[α]D27 = +6.5 (c 1.5, CHCl3)Absolute configuration: (7S)Source of chirality: asymmetric synthesis
(9S)-9,11,11,12,12-Pentamethyl-5-vinyl-2,4,10-trioxa-11-silatridecaneC16H34O3Si[α]D27 = +8.9 (c 1.0, CHCl3)Absolute configuration: (9S)Source of chirality: asymmetric synthesis
(2S)-6-(Methoxymethoxy)oct-7-en-2-olC10H20O3[α]D27 = +4.6 (c 0.4, CHCl3)Absolute configuration: (2S)Source of chirality: asymmetric synthesis
(3R)-(2R)-6-(Methoxymethoxy)oct-7-en-2-yl 3-((tert-butyldimethylsilyl)oxy)hex-5-enoateC22H42O5Si[α]D27 = −20.1 (c 0.74, CHCl3)Absolute configuration: (2R,3R)Source of chirality: asymmetric synthesis
(4R,12R)-4-((tert-Butyldimethylsilyl)oxy)-8-(methoxymethoxy)-12-methyloxacyclododec-6-en-2-oneC20H38O5Si[α]D27 = −1.3 (c 0.48, CHCl3)Absolute configuration: (4R,12R)Source of chirality: asymmetric synthesis
(4R,12R)-4,8-Dihydroxy-12-methyloxacyclododec-6-en-2-oneC12H20O4[α]D27 = −8.2 (c 0.96, CHCl3)Absolute configuration: (4R,12R)Source of chirality: asymmetric synthesis
Dendrodolide LC12H20O4[α]D27 = +8.6 (c 0.2, CHCl3)Absolute configuration: (4R,12R)Source of chirality: asymmetric synthesis
Journal: Tetrahedron: Asymmetry - Volume 27, Issue 6, 1 April 2016, Pages 254–260