| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346612 | 1500359 | 2012 | 6 صفحه PDF | دانلود رایگان |
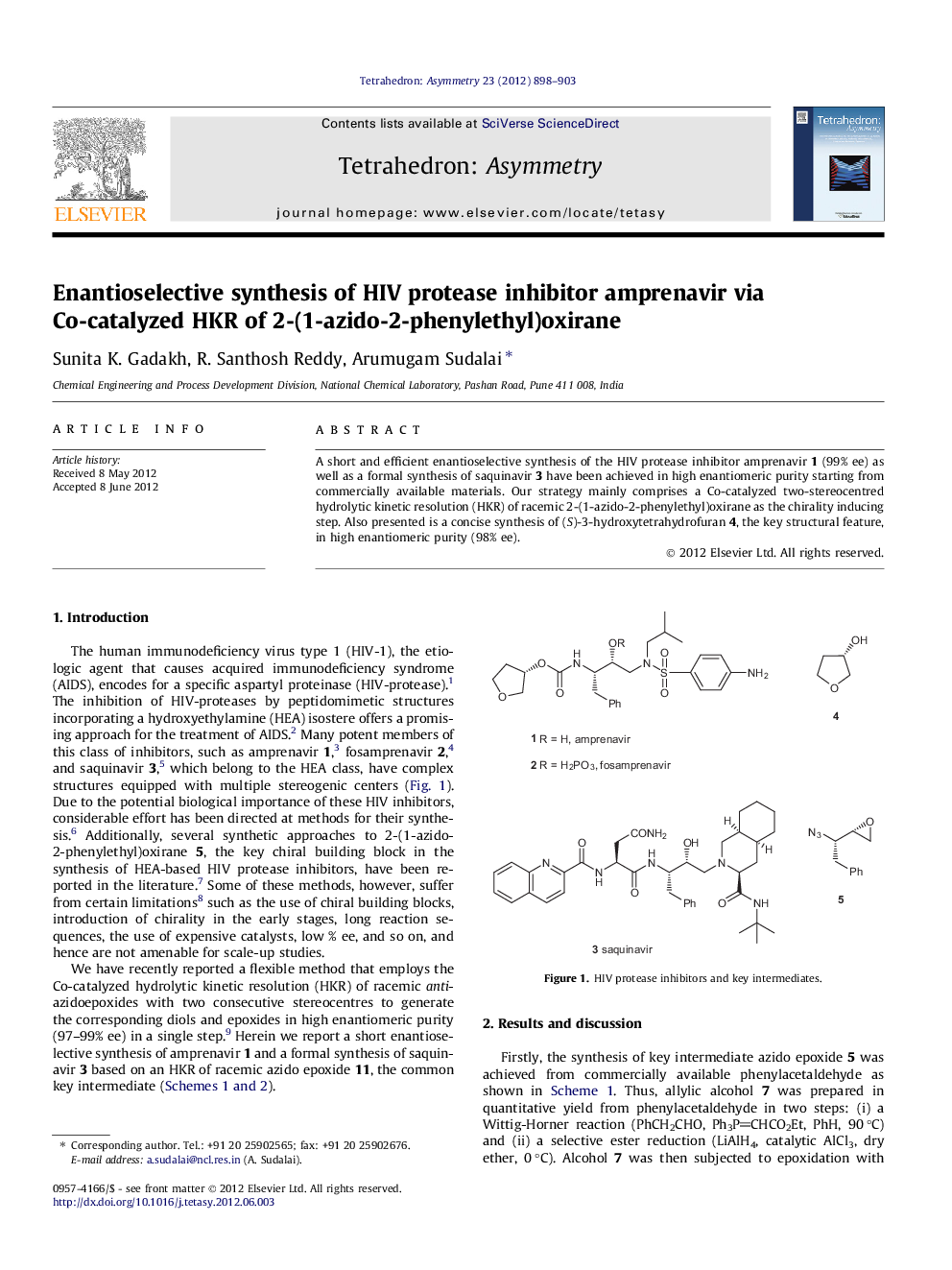
A short and efficient enantioselective synthesis of the HIV protease inhibitor amprenavir 1 (99% ee) as well as a formal synthesis of saquinavir 3 have been achieved in high enantiomeric purity starting from commercially available materials. Our strategy mainly comprises a Co-catalyzed two-stereocentred hydrolytic kinetic resolution (HKR) of racemic 2-(1-azido-2-phenylethyl)oxirane as the chirality inducing step. Also presented is a concise synthesis of (S)-3-hydroxytetrahydrofuran 4, the key structural feature, in high enantiomeric purity (98% ee).
Figure optionsDownload as PowerPoint slide
(2S, 3S)-N-Isobutyl-N-(2-hydroxy-3-azido-4-phenylbutyl)-P-nitrobenzenesulfonylamideC20H25N5O5S[α]D25=-5.3 (c 1, CHCl3)Source of chirality: hydrolytic kinetic resolutionAbsolute configuration: (S,R)ee = 99%
2-((R)-Oxiran-2-yl)ethyl 4-methylbenzenesulfonateC11H14O4S[α]D20=-13.5 (c 1, CHCl3)Source of chirality: hydrolytic kinetic resolutionAbsolute configuration: (R)
2(S)-[1’(S)-azido-2-phenylethyl]oxiraneC10H11N3OEe 99% determined by chiral HPLC[α]D20=+13.1 (c 1, CHCl3)Source of chirality: Two stereocentred Hydrolytic Kinetic ResolutionAbsolute configuration: (S,S)
(2R,3R)-3-Azido-4-phenylbutane-1,2-diolC10H13N3O2Ee 98% determined by chiral HPLC[α]D20=-30.8 (c 1, CHCl3)Source of chirality: Two stereocentred Hydrolytic Kinetic ResolutionAbsolute configuration: (R,R)
4-Nitro-N-((2R(syn),3S)-2-hydroxy-4-phenyl-3-((S)-tetrahydrofuran-3-yloxycarbonylamino)-butyl)-N-isobutylbenzenesulfonamideC25H33N3O8SEe 99% determined by chiral HPLC[α]D20=+15.5 (c 1, CHCl3)Source of chirality: Two stereocentred Hydrolytic Kinetic ResolutionAbsolute configuration: (S,S,R)
2-(3(S)-Azido-2(R)-hydroxy-4-phenylbutyl)-N-tert-butyldecahydro-(4aS,8aS) isoquinoline-3(S)-carbaxomideC24H37N5O2Ee 99% determined by chiral HPLC[α]D20=-75.5 (c 1, CHCl3)Source of chirality: Two stereocentred Hydrolytic Kinetic ResolutionAbsolute configuration: (S,R,S,S,S)
(S)-3-HydroxytetrahydrofuranC4H8O2[α]D20=-17.2 (c 1, MeOH)Source of chirality: Hydrolytic Kinetic ResolutionAbsolute configuration: (S)
Journal: Tetrahedron: Asymmetry - Volume 23, Issues 11–12, 30 June 2012, Pages 898–903