| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346644 | 980272 | 2010 | 8 صفحه PDF | دانلود رایگان |
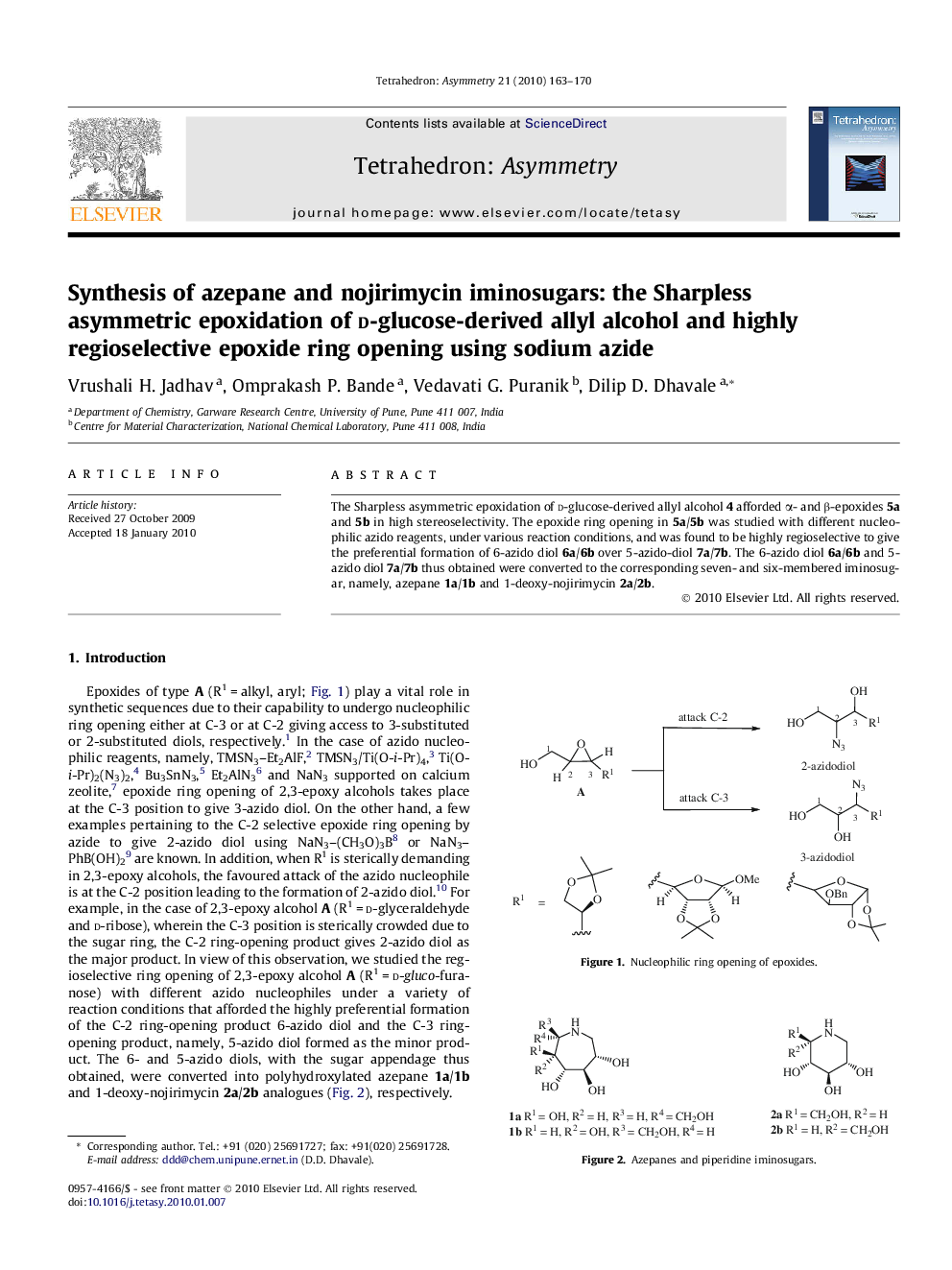
The Sharpless asymmetric epoxidation of d-glucose-derived allyl alcohol 4 afforded α- and β-epoxides 5a and 5b in high stereoselectivity. The epoxide ring opening in 5a/5b was studied with different nucleophilic azido reagents, under various reaction conditions, and was found to be highly regioselective to give the preferential formation of 6-azido diol 6a/6b over 5-azido-diol 7a/7b. The 6-azido diol 6a/6b and 5-azido diol 7a/7b thus obtained were converted to the corresponding seven- and six-membered iminosugar, namely, azepane 1a/1b and 1-deoxy-nojirimycin 2a/2b.
Figure optionsDownload as PowerPoint slide
3-O-Benzyl-5,6-dideoxy-1,2-O-isopropylidene-α-d-xylo-hept-5-eno-1,4-furanoseC17H22O5Ee = 100%[α]D25=-61.4 (c 0.01, CHCl3)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R)
3-O-Benzyl-5,6-anhydro-1,2-O-isopropylidene-αd-glycero-l-ido-hepto-1,4-furanoseC17H22O6Ee = 100%[α]D25=-51.8 (c 0.005, CHCl3)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4S,5S,6R)
3-O-Benzyl-5,6-anhydro-1,2-O-isopropylidene-β-l-glycero-d-gluco-hepto-1,4-furanoseC17H22O6Ee = 100%[α]D25=-44.9 (c 0.08, CHCl3)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4S,5R,6S)
6-Azido-3-O-benzyl-6-deoxy-1,2-O-isopropylidene-β-l-glycero-l-ido-hepto-1,4-furanoseC17H23N3O6Ee = 100%[α]D25=-35.1 (c 0.07, CHCl3)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R,5S,6S)
6-Azido-3-O-benzyl-6-deoxy-1,2-O-isopropylidene-α-d-glycero-d-gluco-hepto-1,4-furanoseC17H23N3O6Ee = 100%[α]D25=-28.7 (c 0.018, CHCl3)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R,5R,6R)
5-Azido-3-O-benzyl-5-deoxy-1,2-O-isopropylidene-α-d-glycero-d-gluco-hepto-1,4-furanoseC17H23N3O6Ee = 100%[α]D25=-71.0 (c 0.0012, CHCl3)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R,5R,6S)
5-Azido-3-O-benzyl-5-deoxy-1,2-O-isopropylidene-β-l-glycero-l-ido-hepto-1,4-furanoseC17H23N3O6Ee = 100%[α]D25=+32.0 (c 0.001, CHCl3)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R,5S,6R)
6-(Benzoxycarbonylamino)-3,5,7-trihydroxy-6-deoxy-1,2-O-isopropylidene-β-l-glycero-l-ido-hepto-1,4-furanoseC18H25NO8Ee = 100%[α]D25=-10.6 (c 0.56, CHCl3)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R,5S,6S)
6-(Benzoxycarbonylamino)-3,5,7-trihydroxy-6-deoxy-1,2-O-isopropylidene-α-d-glycero-d-gluco-hepto-furanoseC18H25NO8Ee = 100%[α]D25=+11.7 (c 0.51, CHCl3)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R,5R,6R)
5-(Benzoxycarbonylamino)-3,6,7-trihydroxy-5-deoxy-1,2-O-isopropylidene-α-d-glycero-d-gluco-hepto-1,4-furanoseC18H25NO8Ee = 100%[α]D25=-9.1 (c 0.005, CHCl3)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R,5R,6S)
5-(Benzoxycarbonylamino)-3,6,7-trihydroxy-5-deoxy-1,2-O-isopropylidene-β-l-glycero-l-ido-hepto-1,4-furanoseC18H25NO8Ee = 100%[α]D25=-13.3 (c 0.15, CHCl3)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R,5S,6R)
5-(Benzoxycarbonylamino)-3, 6-dihydroxy-5-deoxy-1,2-O-isopropylidene-α-d-gluco-hexo-1,4-furanoseC17H23NO7Ee = 100%[α]D25=-4.2 (c 0.045, CHCl3)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R,5R)
5-(Benzoxycarbonylamino)-3,6-dihydroxy-5-deoxy-1,2-O-isopropylidene-β-l-ido-hexo-1,4-furanoseC17H23NO7Ee = 100%[α]D25=+13.3 (c 0.15, CHCl3)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (1R,2R,3S,4R,5S)
1,6-Dideoxy-1,6-imino-l-glycero-l-ido-heptitol hydrochloride. C7H16ClNO5Ee = 100%[α]D25=-4.8 (c 3.35, MeOH)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (2S,3R,4R,5S,6S)
1,6-Dideoxy-1,6-imino-d-glycero-d-gluco-heptitol hydrochloride. C7H16ClNO5Ee = 100%[α]D25=+15.9 (c 1.26, MeOH)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (2S,3R,4R,5R,6R)
1,5-Dideoxy-1,5-imino-d-glucitolC6H14ClNO4Ee = 100%[α]D25=+47.0 (c 0.12, H2O)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (2S,3R,4R,5R)
1,5-Dideoxy-1,5-imino-l-iditolC6H13NO4Ee = 100%[α]D25=+7.9 (c 0.25, MeOH)Source of chirality: 1,2-O-isopropylidene-α-d-glucofuranoseAbsolute configuration: (2S,3R,4R,5S)
Journal: Tetrahedron: Asymmetry - Volume 21, Issue 2, 22 February 2010, Pages 163–170