| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346684 | 1500366 | 2010 | 7 صفحه PDF | دانلود رایگان |
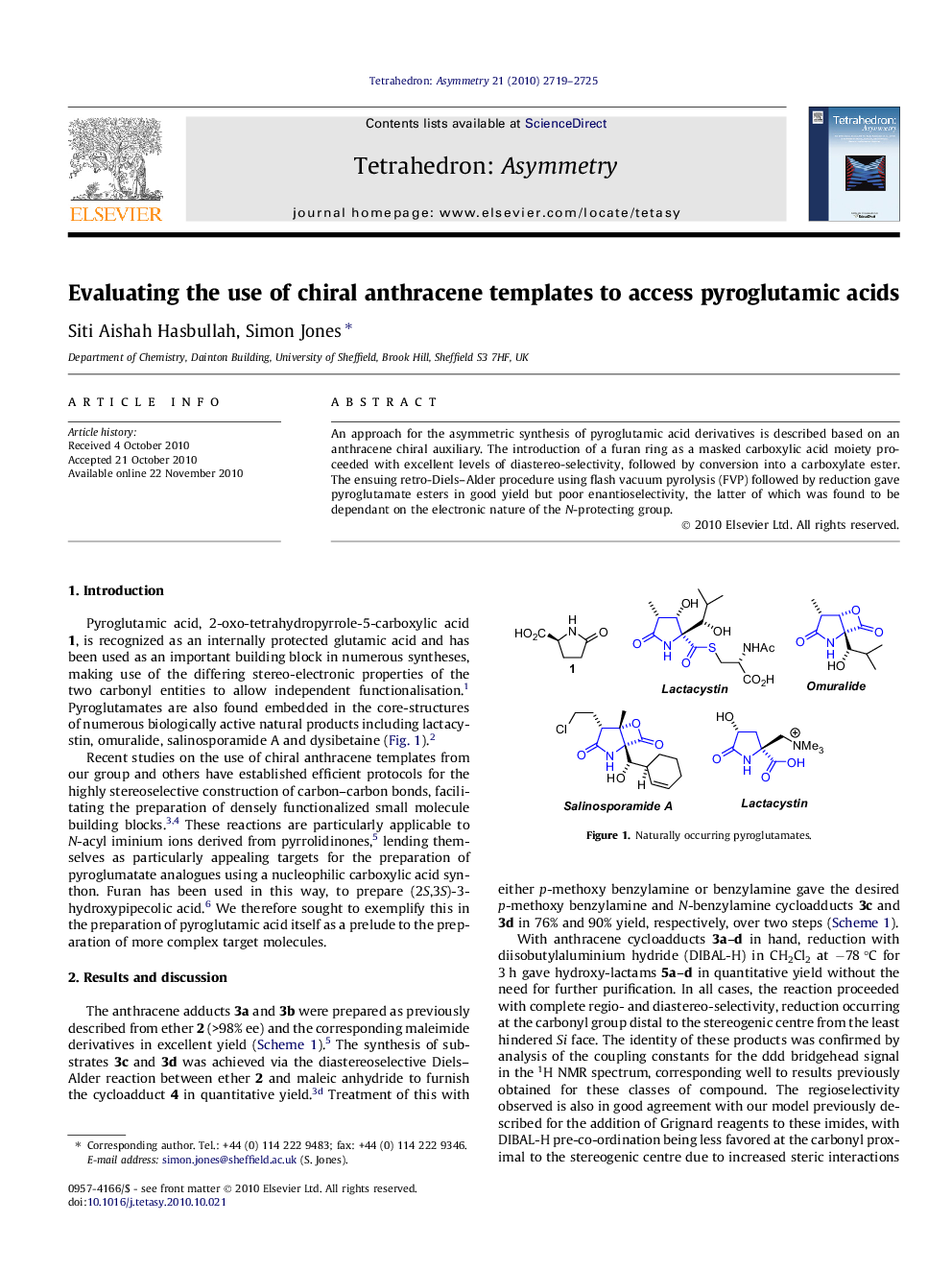
An approach for the asymmetric synthesis of pyroglutamic acid derivatives is described based on an anthracene chiral auxiliary. The introduction of a furan ring as a masked carboxylic acid moiety proceeded with excellent levels of diastereo-selectivity, followed by conversion into a carboxylate ester. The ensuing retro-Diels–Alder procedure using flash vacuum pyrolysis (FVP) followed by reduction gave pyroglutamate esters in good yield but poor enantioselectivity, the latter of which was found to be dependant on the electronic nature of the N-protecting group.
Figure optionsDownload as PowerPoint slide
(3aS,9aS)-3a,4,9,9a-Tetrahydro-4-[(1S)-1-methoxyethyl]-2-(N-4′-methoxyphenylmethyl)-4,9-[1′,2′]benzeno-1H-benzo[f]isoindole-1,3-(2H)-dioneC29H27NO4[α]D = −42 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3aR,9aR) (1R)
(3aS,9aS)-3a,4,9,9a-Tetrahydro-4-[(1S)-1-methoxyethyl]-2-(N-phenylmethyl)-4,9-[1′,2′]benzeno-1H-benzo[f]isoindole-1,3-(2H)-dioneC28H25NO3[α]D = −36 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3aR,9aR) (1R)
(3S,3aS,9aS)-9-[(1S)-1-Methoxyethyl]-2,3,3a,4,9,9a-hexahydro-3-hydroxy-2-methyl-4,9 [1′,2′]-benzeno-1H-benz[f]isoindol-1-oneC22H23NO3[α]D = −24 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3R,3aR,9aR) (1R)
(3S,3aS,9aS)-9-[(1S)-1-Methoxyethyl]-2,3,3a,4,9,9a-hexahydro-3-hydroxy-2-[N-4′-methoxyphenyl]-4,9 [1′,2′]-benzeno-1H-benz[f]isoindol-1-oneC22H23NO3[α]D = −44 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3R,3aR,9aR) (1R)
(3S,3aS,9aS)-9-[(1S)-1-Methoxyethyl]-2,3,3a,4,9,9a-hexahydro-3-hydroxy-2-[N-4′-methoxyphenylmethyl]-4,9 [1′,2′]-benzeno-1H-benz[f]isoindol-1-oneC29H29NO4[α]D = +8 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3R,3aR,9aR) (1R)
(3S,3aS,9aS)-9-[(1S)-1-Methoxyethyl]-2,3,3a,4,9,9a-hexahydro-3-hydroxy-2-[N-phenylmethyl]-4,9 [1′,2′]-benzeno-1H-benz[f]isoindol-1-oneC28H27NO3[α]D = +9 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3R,3aR,9aR) (1R)
(3aS,9aS,3S)-2,3,3a,4,9,9a-Hexahydro-4-[(1S)-1-methoxyethyl]-3-(2-furanyl)-2-methyl-4,9-[1′,2′]benzeno-1H-benz[f]isoindol-1-oneC26H25NO3[α]D = +59 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3aR,9aR,3R) (1R)
(3aS,9aS,3S)-2,3,3a,4,9,9a-Hexahydro 4-[(1S)-1-methoxyethyl]-3-(2-furanyl)-2-(N-4′-methoxyphenyl)-4,9[1′,2′]benzeno-1H-benz[f]isoindol-1-oneC32H29NO4[α]D = +8 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3aR,9aR,3R) (1R)
(3aS,9aS,3S)-2,3,3a,4,9,9a-Hexahydro 4-[(1S)-1-methoxyethyl]-3-(2-furanyl)-2-(N-4′-methoxyphenylmethyl)-4,9[1′,2′]benzeno-1H-benz[f]isoindol-1-oneC33H31NO4[α]D = +8 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3aR,9aR,3R) (1R)
(3aS,9aS,3S)-2,3,3a,4,9,9a-Hexahydro-4-[(1S)-1-methoxyethyl]-2-(N-phenylmethyl)-3-(2-furanyl)-2-methyl-4,9[1′,2′]benzeno-1H-benz[f]isoindol-1-oneC32H29NO3[α]D = +21 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3aR,9aR,3R) (1R)
(3aS,9aS,3S)-2,3,3a,4,9,9a-Hexahydro-4-[(1S)-1-methoxyethyl]-2-methyl-4,9-[1′,2′]benzeno-1H-benz[f]isoindol-1-one-3-carboxylic acid methyl esterC24H25NO4[α]D = +21 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3aR,9aR,3R) (1R)
(3aS,9aS,3S)-2,3,3a,4,9,9a-Hexahydro-4-[(1S)-1-methoxyethyl]-2-(N-4′-methoxyphenyl)-4,9-[1′,2′]benzeno-1H-benz[f]isoindol-1-one-3-carboxylic acid methyl esterC30H29NO5[α]D = +13 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3aR,9aR,3R) (1R)
(3aS,9aS,3S)-2,3,3a,4,9,9a-Hexahydro-4-[(1S)-1-methoxyethyl]-2-(N-4′-methoxyphenylmethyl)-4,9-[1′,2′]benzeno-1H-benz[f]isoindol-1-one-3-carboxylic acid methyl esterC31H31NO5[α]D = +10 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3aR,9aR,3R) (1R)
(3aS,9aS,3S)-2,3,3a,4,9,9a-Hexahydro-4-[(1S)-1-methoxyethyl]-2-(N-phenylmethyl)-4,9-[1′,2′]benzeno-1H-benz[f]isoindol-1-one-3-carboxylic acid methyl esterC30H29NO4[α]D = +2 (c 1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3aR,9aR,3R) (1R)
(S)-1-(4-Methoxyphenyl)-5-oxo-proline methyl esterC13H15NO4Ee 23%[α]D = −6 (c 0.8, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
Journal: Tetrahedron: Asymmetry - Volume 21, Issues 21–22, 25 November 2010, Pages 2719–2725