| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346706 | 1500364 | 2011 | 5 صفحه PDF | دانلود رایگان |
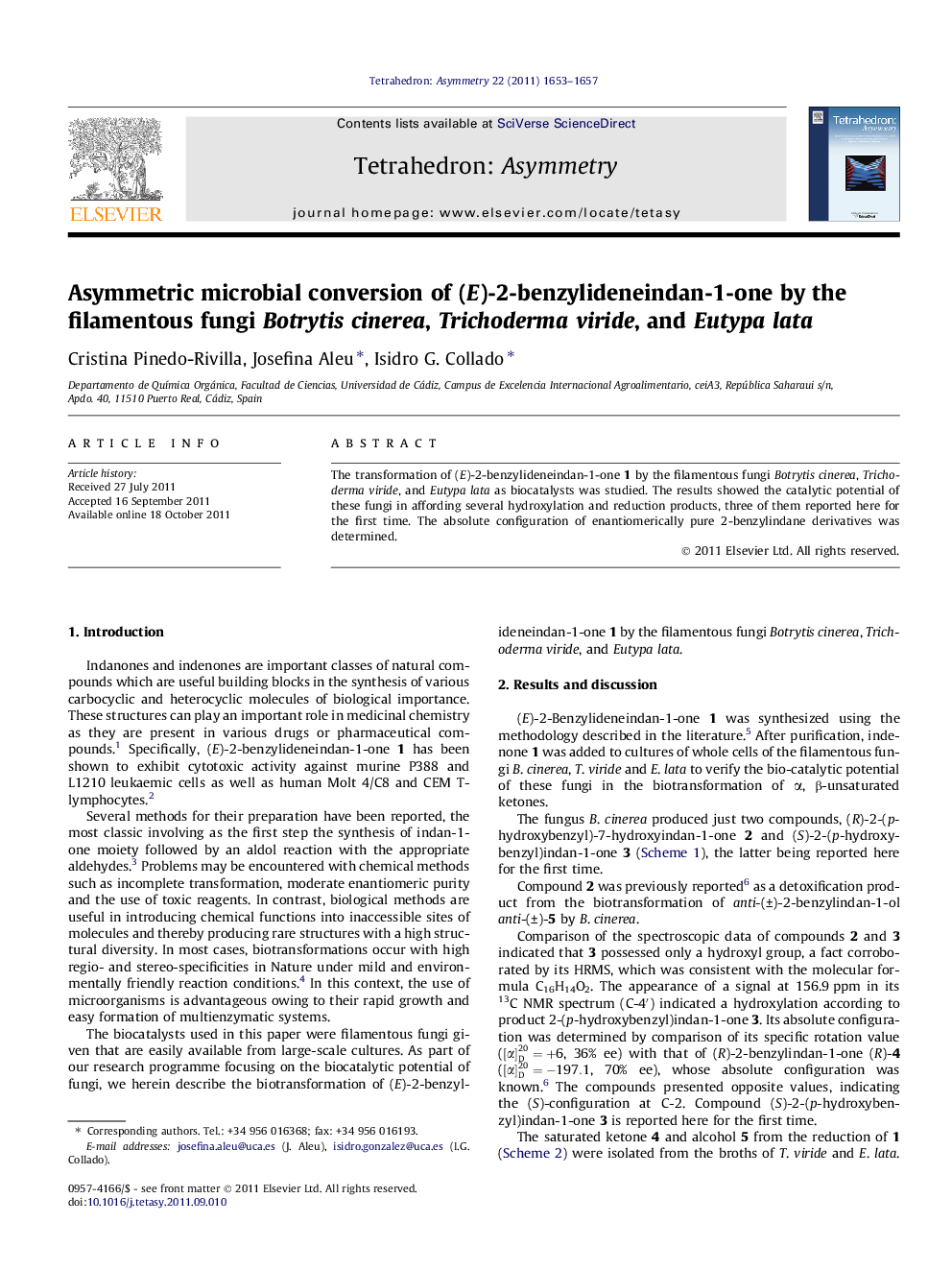
The transformation of (E)-2-benzylideneindan-1-one 1 by the filamentous fungi Botrytis cinerea, Trichoderma viride, and Eutypa lata as biocatalysts was studied. The results showed the catalytic potential of these fungi in affording several hydroxylation and reduction products, three of them reported here for the first time. The absolute configuration of enantiomerically pure 2-benzylindane derivatives was determined.
Figure optionsDownload as PowerPoint slide
(R)-2-(p-Hydroxybenzyl)-7-hydroxyindan-1-oneC16H14O3Ee = 19%[α]D20=−6.5 (c 0.1, MeOH)Source of chirality: microbial transformation by Botrytis cinereaAbsolute configuration: (R)
(S)-2-(p-Hydroxybenzyl)indan-1-oneC16H14O2Ee = 36%[α]D20=+6 (c 0.1, MeOH)Source of chirality: microbial transformation by Botrytis cinereaAbsolute configuration: (S)
(R)-2-Benzylindan-1-oneC16H14OEe = 86%[α]D20=−197.1 (c 0.1, CHCl3)Source of chirality: microbial transformation by Trichoderma virideAbsolute configuration: (R)
(1R,2R)-2-Benzylindan-1-olC16H16ODe = 100%Ee = 99%[α]D20=−5 (c 0.1, CHCl3)Source of chirality: microbial transformation by Trichoderma virideAbsolute configuration: (R,R)
(1S,2R)-2-Benzylindan-1-olC16H16ODe = 100%Ee = 95%[α]D20=−11 (c 0.1, CHCl3)Source of chirality: microbial transformation by Eutypa lataAbsolute configuration: (S,R)
Journal: Tetrahedron: Asymmetry - Volume 22, Issues 16–17, 15 September 2011, Pages 1653–1657