| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346712 | 1500364 | 2011 | 5 صفحه PDF | دانلود رایگان |
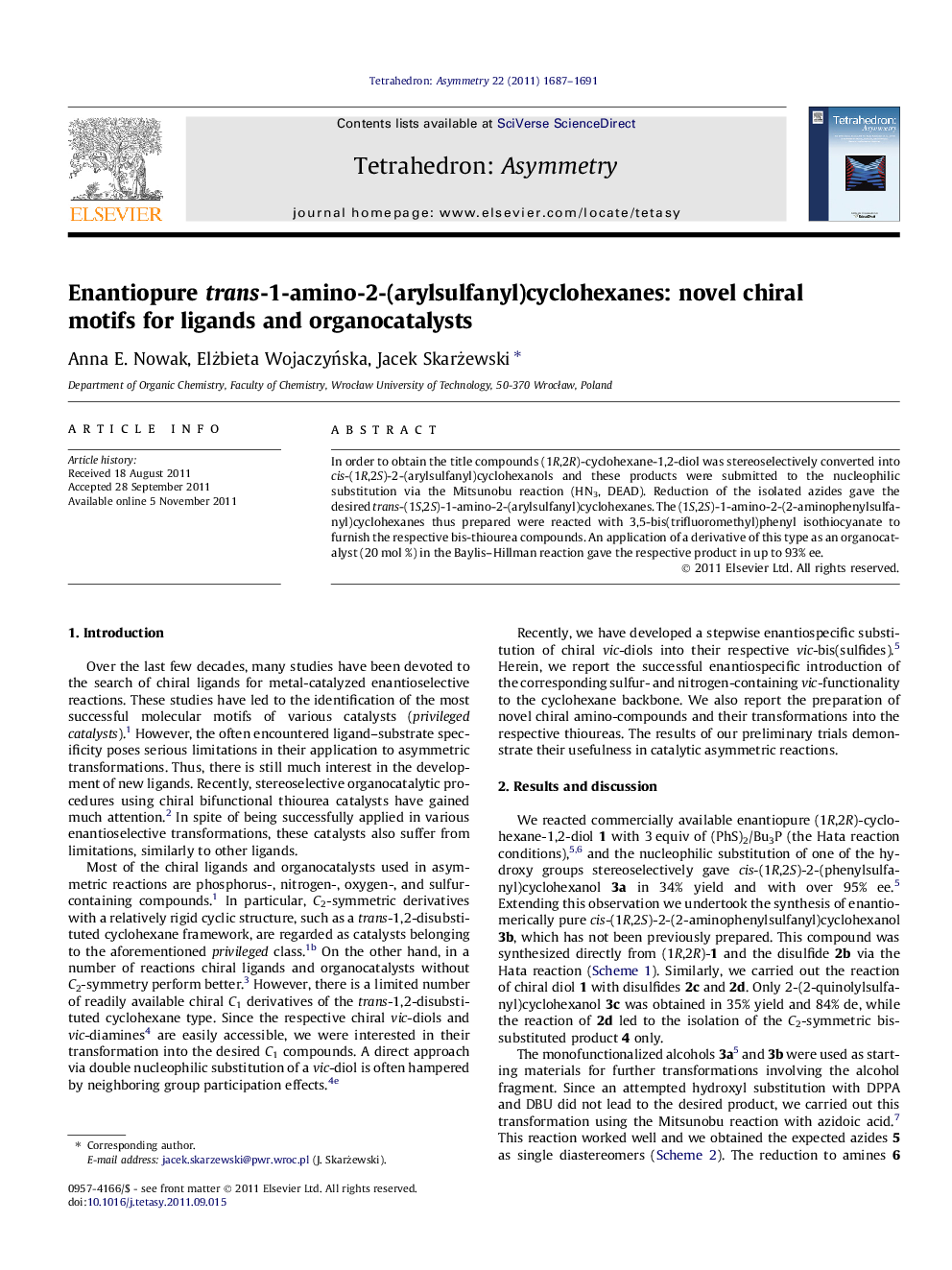
In order to obtain the title compounds (1R,2R)-cyclohexane-1,2-diol was stereoselectively converted into cis-(1R,2S)-2-(arylsulfanyl)cyclohexanols and these products were submitted to the nucleophilic substitution via the Mitsunobu reaction (HN3, DEAD). Reduction of the isolated azides gave the desired trans-(1S,2S)-1-amino-2-(arylsulfanyl)cyclohexanes. The (1S,2S)-1-amino-2-(2-aminophenylsulfanyl)cyclohexanes thus prepared were reacted with 3,5-bis(trifluoromethyl)phenyl isothiocyanate to furnish the respective bis-thiourea compounds. An application of a derivative of this type as an organocatalyst (20 mol %) in the Baylis–Hillman reaction gave the respective product in up to 93% ee.
Catalyst for the Baylis–Hillman Reaction, giving up to 93% ee.Figure optionsDownload as PowerPoint slide
(1R,2S)-2-(2-Aminophenylsulfanyl)cyclohexanolC12H18NOSDe >95% (by 1H NMR)[α]D20=−52.1 (c 0.86, CH2Cl2) >95% eeSource of chirality: stereoselective nucleophilic substitutionAbsolute configuration: (1R,2S) (by chemical correlation)
(1S,2S)-1,2-Bis(2-pirydylsulfanyl)cyclohexaneC16H19N2S2De >98% (by 1H NMR)[α]D20=−40.0 (c 1.046, CH2Cl2) >95% eeSource of chirality: stereoselective nucleophilic substitutionAbsolute configuration: (1S,2S) (by chemical correlation)
(1S,2S)-trans-2-(Phenylsulfanyl)cyclohexylamineC12H17NSDe >98% (by 1H NMR)[α]D20=+84.2 (c 0.38, CH2Cl2), ee >95%Source of chirality: stereoselective nucleophilic substitutionAbsolute configuration: (1S,2S) (by chemical correlation)
3-(2-{[(1S,2R)-2-Hydroxycyclohexyl]sulfanyl}phenyl)-1-phenylthioureaC19H22N2OS2De >98% (by 1H NMR)[α]D20=+18.4 (c 3.16, CH2Cl2) >95% eeSource of chirality: enantiopure substrateAbsolute configuration: (1R,2S) (by chemical correlation)
1-[3,5-Bis(trifluoromethyl)phenyl]-3-(2-{[(1S,2R)-2-hydroxycyclohexyl]sulfanyl}phenyl)thioureaC21H21F6N2OS2De >98% (by 1H NMR)[α]D20=+90.9 (c 0.66, CH2Cl2) >95% eeSource of chirality: enantiopure substrateAbsolute configuration: (1R,2S) (by chemical correlation)
1-[3,5-Bis(trifluoromethyl)phenyl]-3-[(1S,2S)-2-(phenylsulfanyl)cyclohexyl]thioureaC21H21F6N2S2De >98% (by 1H NMR)[α]D20=+110.5 (c 0.19, CH2Cl2) >95% eeSource of chirality: enantiopure substrateAbsolute configuration: (1S,2S) (by chemical correlation)
1-Phenyl-3-[(1S,2S)-2-({2-[(phenylcarbamothioyl)amino]phenyl}sulfanyl)cyclohexyl]thioureaC26H28N4S3De >98% (by 1H NMR)[α]D20=+46.3 (c 0.8, CH2Cl2) >95% eeSource of chirality: enantiopure substrateAbsolute configuration: (1S,2S) (by chemical correlation)
(1S,2S)-2-(2-Aminophenylsulfanyl)cyclohexylamineC12H19N2SDe >98% (by 1H NMR)[α]D20=+54.2 (0.24, CH2Cl2) >95% eeSource of chirality: stereoselective nucleophilic substitutionAbsolute configuration: (1S,2S) (by chemical correlation)
1-[3,5-Bis(trifluoromethyl)phenyl]-3-[(1S,2S)-2-{[2-({[3,5-bis(trifluoromethyl)phenyl]carbamothioyl}amino)phenyl]sulfanyl}-cyclohexyl]thioureaC30H25S3N4F12De >98% (by 1H NMR)[α]D20=+3..6 (c 0.41, CH2Cl2) >95% eeSource of chirality: enantiopure substrateAbsolute configuration: (1S,2S) (by chemical correlation)
Journal: Tetrahedron: Asymmetry - Volume 22, Issues 16–17, 15 September 2011, Pages 1687–1691