| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346714 | 1500364 | 2011 | 5 صفحه PDF | دانلود رایگان |
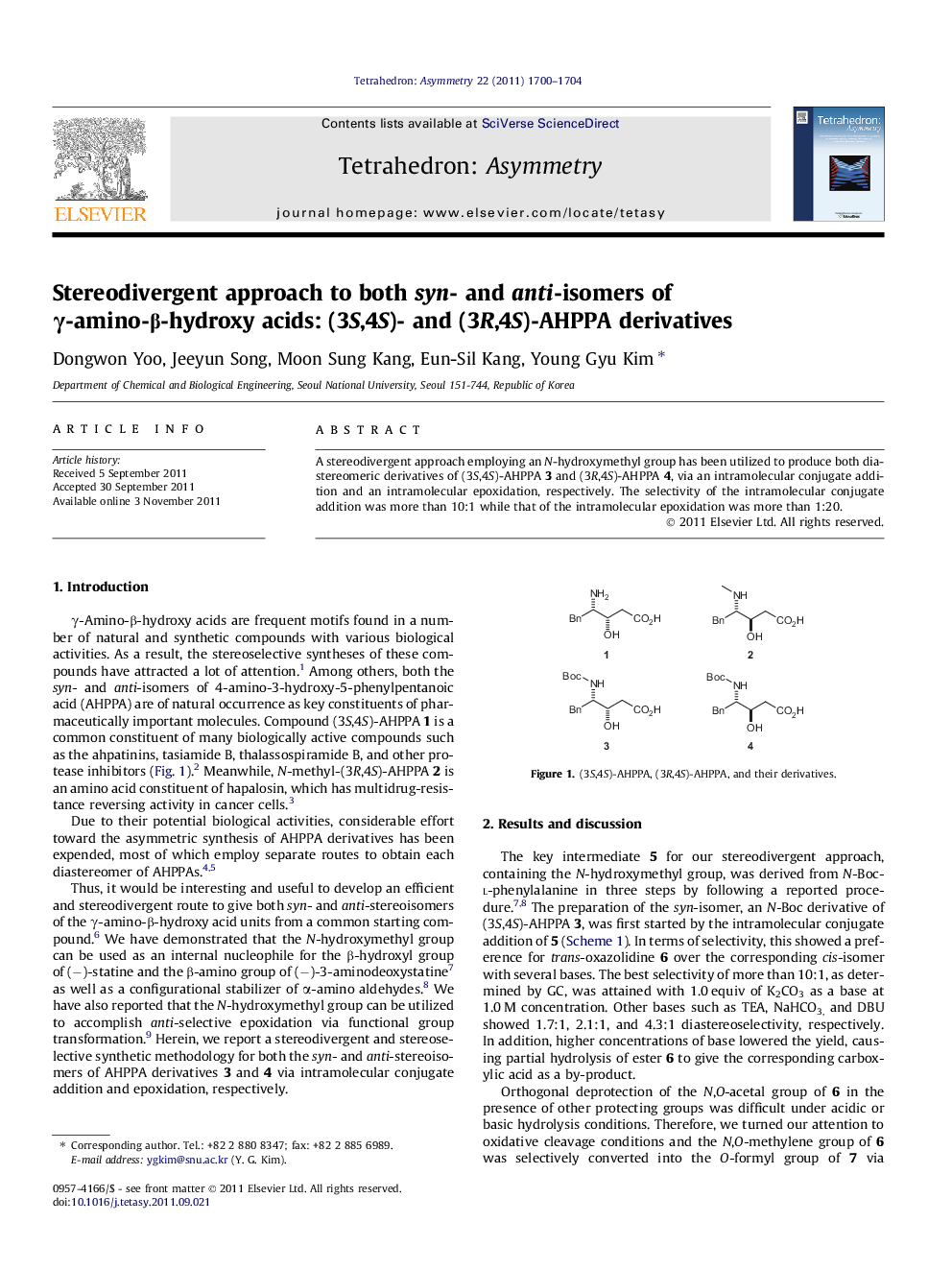
A stereodivergent approach employing an N-hydroxymethyl group has been utilized to produce both diastereomeric derivatives of (3S,4S)-AHPPA 3 and (3R,4S)-AHPPA 4, via an intramolecular conjugate addition and an intramolecular epoxidation, respectively. The selectivity of the intramolecular conjugate addition was more than 10:1 while that of the intramolecular epoxidation was more than 1:20.
Figure optionsDownload as PowerPoint slide
Methyl (2E,4S)-4-(N-tert-butoxycarbonyl-N-hydroxymethyl)amino-5-phenylpent-2-enoateC18H23NO5[α]D19=-36.7 (c 1.74, CHCl3)Source of chirality: l-phenylalanineAbsolute configuration: (4S)
Methyl (2E,4S)-4-(N-tert-butoxycarbonyl-N-acetoxymethyl)amino-5-phenylpent-2-enoateC20H27NO6[α]D19=-34.9 (c 0.32, CHCl3)Source of chirality: l-phenylalanineAbsolute configuration: (4S)
Methyl (3S,4S)-4-(N-tert-Buthoxycarbonyl)amino-3-hydroxy-5-phenylpentanoateC17H25NO5[α]D20=-36.5 (c 0.10, CH3OH)Source of chirality: l-phenylalanine and intramolecular conjugate additionAbsolute configuration: (3S,4S)
Methyl (3R,4S)-4-(N-tert-buthoxycarbonyl)amino-3-hydroxy-5-phenylpentanoateC17H25NO5[α]D20=-19.9 (c 0.20, CH3OH)Source of chirality: l-phenylalanine and intramolecular epoxidationAbsolute configuration: (3R,4S)
(3S,4S)-4-(tert-Butoxycarbonyl)amino-3-hydroxy-5-phenylpentanoic acidC16H23NO5[α]D20=-36.8 (c 0.050, CH3OH)Source of chirality: l-phenylalanine and intramolecular conjugate additionAbsolute configuration: (3S,4S)
(3R,4S)-4-(tert-Butoxycarbonyl)amino-3-hydroxy-5-phenylpentanoic acidC16H23NO5[α]D20=-17.9 (c 0.024, CH3OH)Source of chirality: l-phenylalanine and intramolecular epoxidationAbsolute configuration: (3R,4S)
Journal: Tetrahedron: Asymmetry - Volume 22, Issues 16–17, 15 September 2011, Pages 1700–1704