| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346723 | 1500335 | 2015 | 5 صفحه PDF | دانلود رایگان |
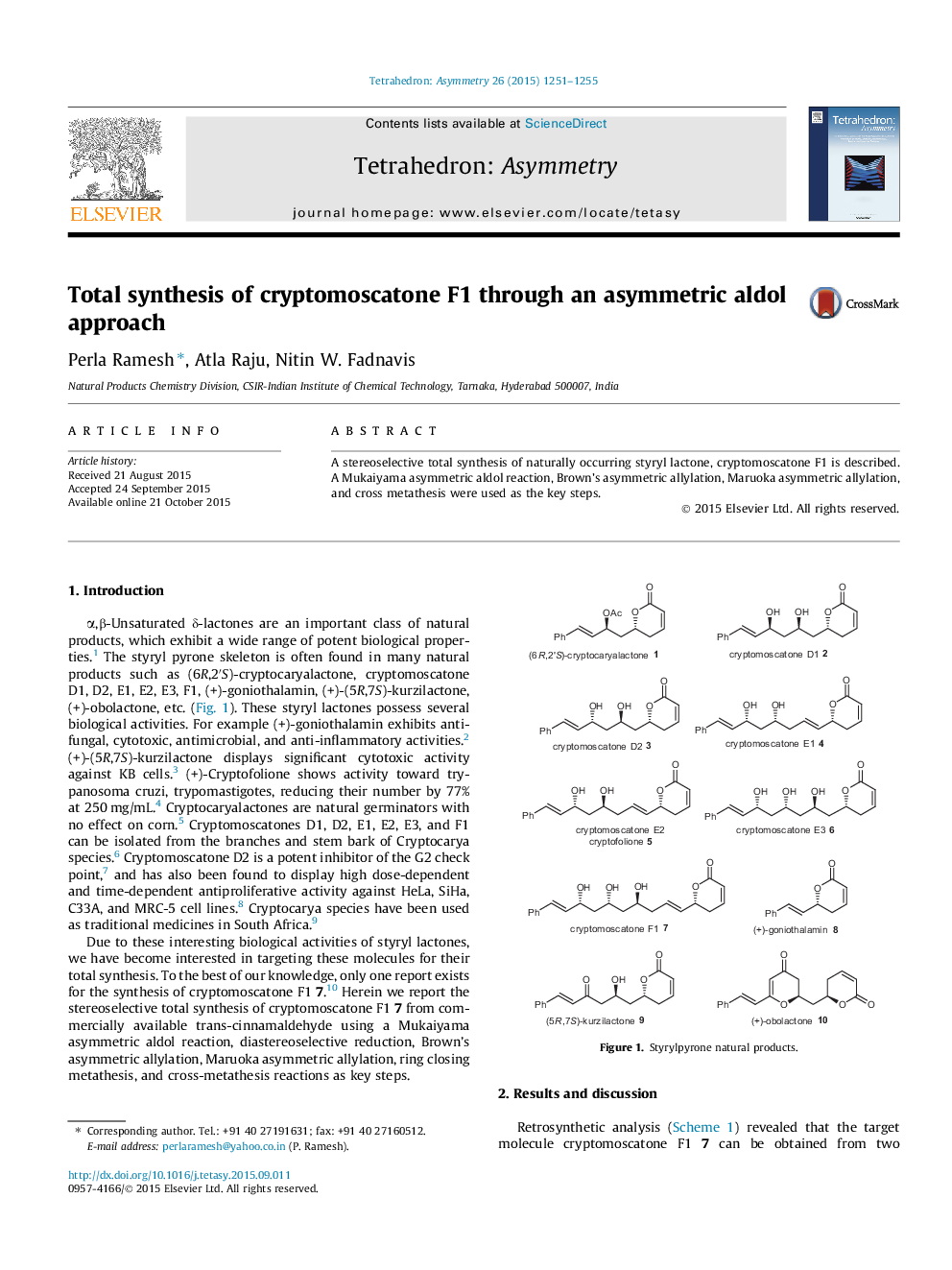
A stereoselective total synthesis of naturally occurring styryl lactone, cryptomoscatone F1 is described. A Mukaiyama asymmetric aldol reaction, Brown’s asymmetric allylation, Maruoka asymmetric allylation, and cross metathesis were used as the key steps.
Figure optionsDownload as PowerPoint slide
(R,E)-Ethyl 5-hydroxy-3-oxo-7-phenylhept-6-enoateC15H18O4[α]D25 = +14.1 (c 1.0, CHCl3)Absolute configuration: (R,E)Source of chirality: asymmetric synthesis
Ethyl 2-((4S,6R)-2,2-dimethyl-6-((E)-styryl)-1,3-dioxan-4-yl)acetateC18H24O4[α]D25 = +16.4 (c 0.4, CHCl3)Absolute configuration: (4S,6R,E)Source of chirality: asymmetric synthesis
(S)-1-((4R,6R)-2,2-Dimethyl-6-((E)-styryl)-1,3-dioxan-4-yl)pent-4-en-2-olC19H26O3[α]D25 = +21.4 (c 1.0, CHCl3)Absolute configuration: (S,4R,6R,E)Source of chirality: asymmetric synthesis
(3R,5R,7S,E)-1-Phenyldeca-1,9-diene-3,5,7-triolC16H22O3[α]D25 = +43.9 (c 1.0, CHCl3)Absolute configuration: (3R,5R,7S,E)Source of chirality: asymmetric synthesis
(R,E)-1-((4S,6S)-6-Allyl-2,2-dimethyl-1,3-dioxan-4-yl)-4-phenylbut-3-en-2-olC19H26O3[α]D25 = +22.9 (c 0.5, CHCl3)Absolute configuration: (R,4S,6R,E)Source of chirality: asymmetric synthesis
(R,E)-1-Phenylhexa-1,5-dien-3-olC12H14O[α]D25 = +22.2 (c 2.0, CHCl3)Absolute configuration: (R,E)Source of chirality: asymmetric synthesis
(R,E)-1-Phenylhexa-1,5-dien-3-yl acrylateC15H16O2[α]D25 = −80.9 (c 1.0, CHCl3)Absolute configuration: (R,E)Source of chirality: asymmetric synthesis
(R,E)-6-Styryl-5,6-dihydro-2H-pyran-2-oneC13H12O2[α]D25 = −169.3 (c 1.1, CHCl3)Absolute configuration: (R,E)Source of chirality: asymmetric synthesis
(R)-6-Vinyl-5,6-dihydro-2H-pyran-2-oneC7H8O2[α]D25 = +90.1 (c 1.0, CHCl3)Absolute configuration: (R)Source of chirality: asymmetric synthesis
(R)-6-((1E,4S,6R,8R,9E)-4,6,8-Trihydroxy-10-phenyldeca-1,9-dien-1-yl)-5,6-dihydro-2H-pyran-2-oneC21H26O5[α]D25 = +34.1 (c 0.9, CHCl3)Absolute configuration: (R,4S,6R,8R,1E,9E)Source of chirality: asymmetric synthesis
Journal: Tetrahedron: Asymmetry - Volume 26, Issues 21–22, 1 December 2015, Pages 1251–1255