| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346773 | 980278 | 2010 | 6 صفحه PDF | دانلود رایگان |
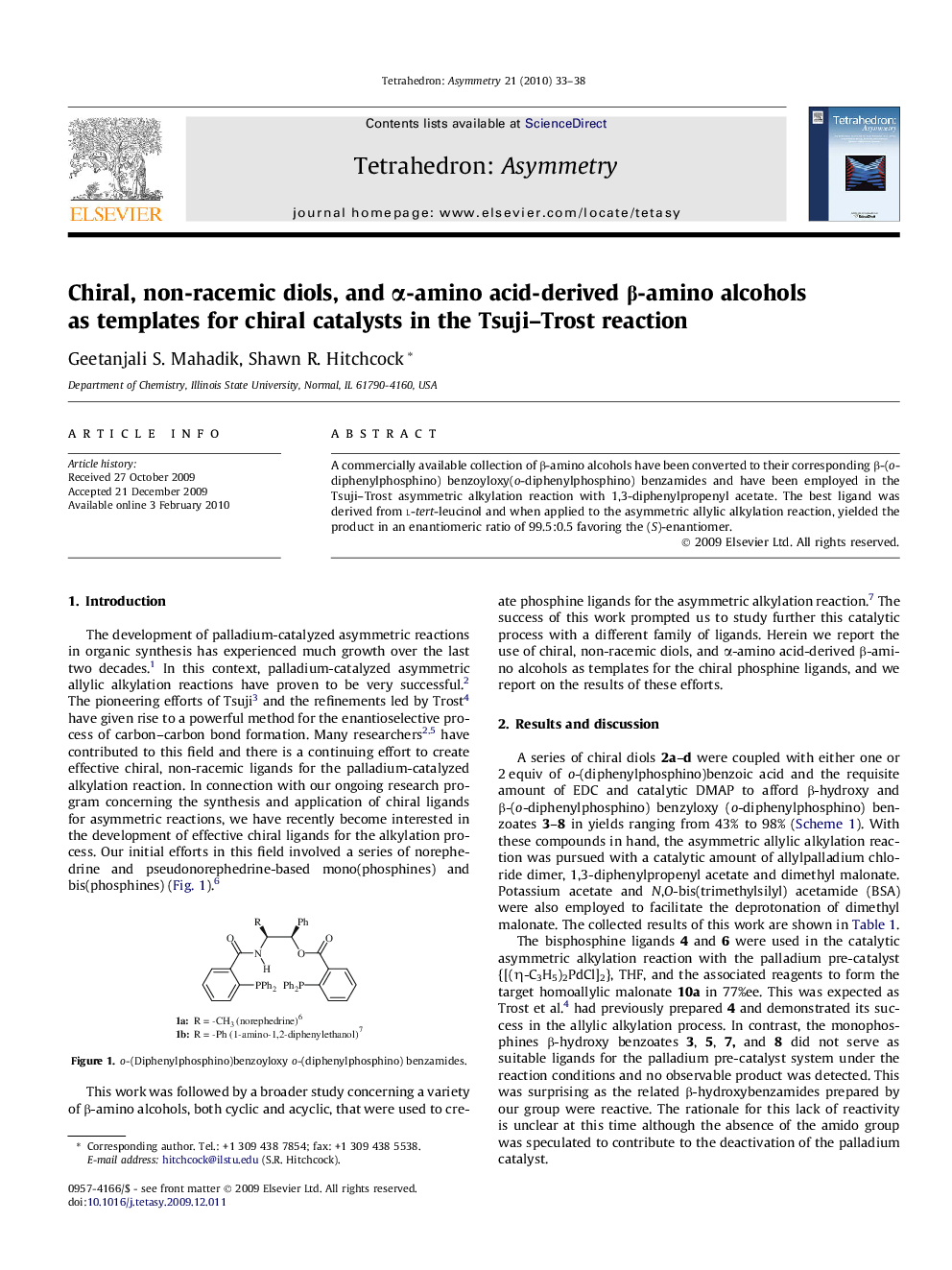
A commercially available collection of β-amino alcohols have been converted to their corresponding β-(o-diphenylphosphino) benzoyloxy(o-diphenylphosphino) benzamides and have been employed in the Tsuji–Trost asymmetric alkylation reaction with 1,3-diphenylpropenyl acetate. The best ligand was derived from l-tert-leucinol and when applied to the asymmetric allylic alkylation reaction, yielded the product in an enantiomeric ratio of 99.5:0.5 favoring the (S)-enantiomer.
Figure optionsDownload as PowerPoint slide
(1R,2R)-2-Hydroxy-1,2-diphenyl 2-(diphenylphosphino)benzoateC33H27O3P[α]D24=+41.9 (c 0.80, CHCl3)Source of chirality: (R,R)-hydrobenzoinAbsolute configuration: (1R,2R)
(1R,2R)-1,2-Diphenylethane-1,2-diyl bis(2-(diphenylphosphino)benzoate)C52H40O4P2[α]D23=+104.4 (c 0.10, CHCl3)Source of chirality: (R,R)-hydrobenzoinAbsolute configuration: (1R,2R)
(1R,2R)-2-Hydroxy-1,2-di(naphthalen-1-yl)ethyl 2-(diphenylphosphino)benzoateC41H31O3P[α]D24=+121.5 (c 0.55, CHCl3)Source of chirality: (R,R)-1,2-di(1-naphthyl)-1,2-ethanediolAbsolute configuration: (1R,2R)
(1R,2R)-1,2-Di(napthalen-1-yl)ethane-1,2-diyl bis(2-(diphenylphosphino)benzoate)C60H44O4P2[α]D23=+276.0 (c 0.21, CHCl3)Source of chirality: (R,R)-(+)-1,2-di(1-naphthyl)-1,2-ethanediolAbsolute configuration: (1R,2R)
(S)-2-Hydroxy-1,2,2-triphenylethyl 2-(diphenylphosphino)benzoateC39H31O3P[α]D23=-131.7 (c 0.44, CHCl3)Source of chirality: (S)-1,1,2-triphenyl-1,2-ethanediolAbsolute configuration: (S)
(R)-2-Hydroxy-2-phenylethyl 2-(diphenylphosphino)benzoateC27H23O3P[α]D23=+2.22 (c 1.68, CHCl3)Source of chirality: (R)-1-phenyl-1,2-ethanediolAbsolute configuration: (R)
(1R,2R)-2-Acetoxy-1,2-diphenylethyl 2-(diphenylphosphino)benzoateC35H29O4P[α]D23=+19.6 (c 0.58, CHCl3)Source of chirality: (1R,2R)-2-hydroxy-1,2-diphenyl 2-(diphenylphosphino)benzoateAbsolute configuration: (1R,2R)
(S)-2-(2-(Diphenylphosphino)benzamido)-3-methylbutyl 2-(diphenylphosphino)benzoateC43H39NO3P2[α]D23=-8.1 (c 0.20, CHCl3)Source of chirality: (S)-2-amino-3-methyl-1-butanolAbsolute configuration: (S)
(S)-2-(2-(Diphenylphosphino)benzamido)-2-phenylethyl 2-(diphenylphosphino)benzoateC46H37NO3P2[α]D23=-6.3 (c 0.35, CHCl3)Source of chirality: (S)-phenylglycinolAbsolute configuration: (S)
(S)-2-(2-(Diphenylphosphino)benzamido)-3,3-dimethylbutyl 2-(diphenylphosphino)benzoateC44H41NO3P2[α]D23=-3.2 (c 0.40, CHCl3)Source of chirality: (S)-2-amino-3,3-dimethyl-1-butanolAbsolute configuration: (S)
Journal: Tetrahedron: Asymmetry - Volume 21, Issue 1, 29 January 2010, Pages 33–38