| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346808 | 980280 | 2011 | 5 صفحه PDF | دانلود رایگان |
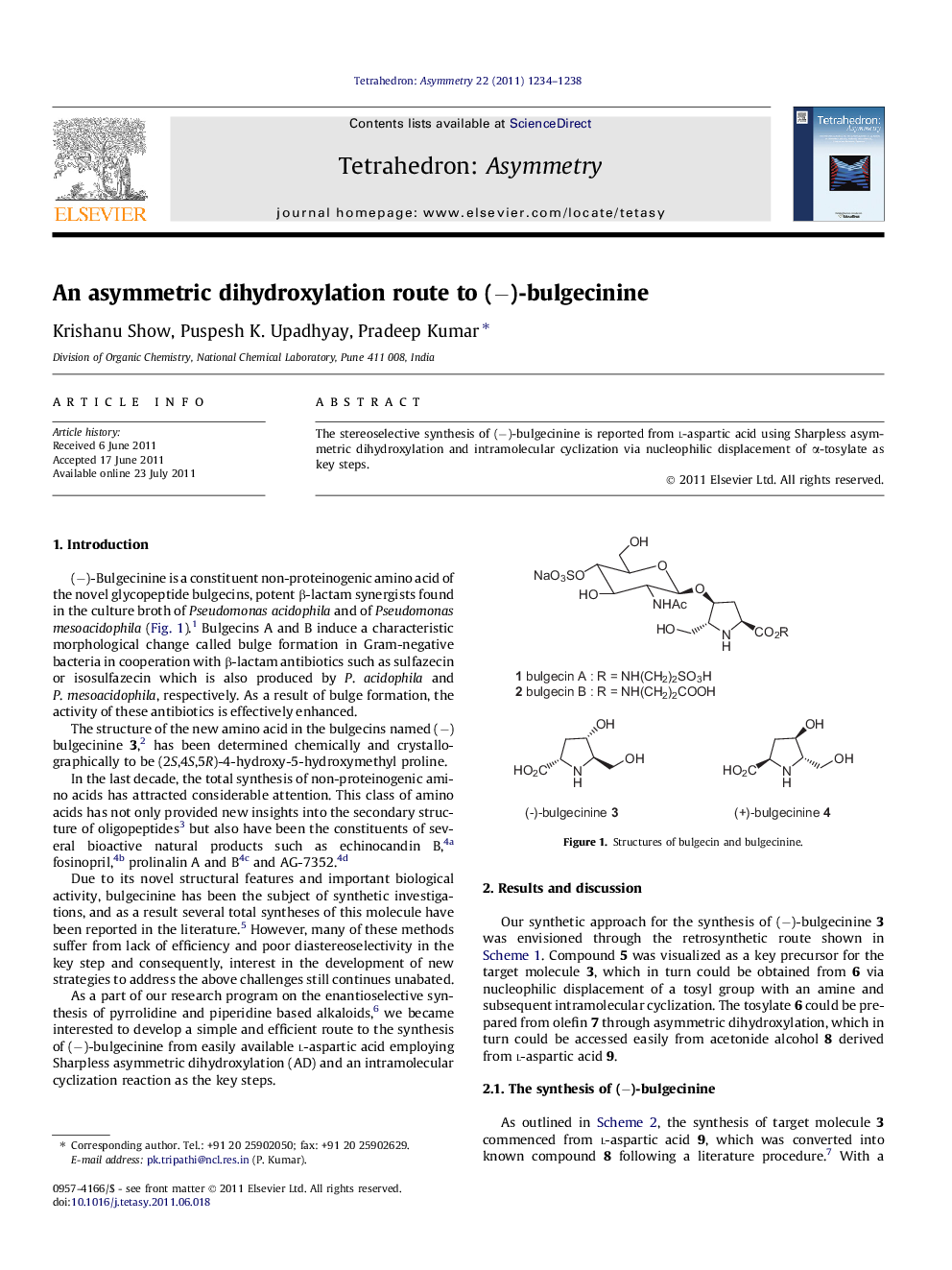
The stereoselective synthesis of (−)-bulgecinine is reported from l-aspartic acid using Sharpless asymmetric dihydroxylation and intramolecular cyclization via nucleophilic displacement of α-tosylate as key steps.
Figure optionsDownload as PowerPoint slide
(S,E)-Ethyl 5-((tert-butoxycarbonyl)amino)-6-((tert-butyldiphenylsilyl)oxy)hex-2-enoateC29H41NO5Si[α]D25=-9.5 (c 1.0, CHCl3)Source of chirality: l-aspartic acidAbsolute configuration : (5S)
(2R,3S,5S)-Ethyl 5-((tert-butoxycarbonyl)amino)-6-((tert-butyldiphenylsilyl)oxy)-2,3-dihydroxyhexanoateC29H43NO7Side: 97%[α]D25=-11.0 (c 1.5, CHCl3)Source of chirality: l-aspartic acid and asymmetric dihydroxylationAbsolute configuration : (2R,3S,5S)
(2R,3S,5S)-Ethyl 5-((tert-butoxycarbonyl)amino)-6-((tert-butyldiphenylsilyl)oxy)-3-hydroxy-2-(tosyloxy)hexanoateC36H49NO9SSi[α]D25=-5.6 (c 4.0, CHCl3)Source of chirality: l-aspartic acid and asymmetric dihydroxylationAbsolute configuration : (2R,3S,5S)
(2S,3S,5S)-Ethyl 5-(((tert-butyldiphenylsilyl)oxy)methyl)-3-hydroxypyrrolidine-2-carboxylateC24H33NO4Si[α]D25=+12.2 (c 0.08, CHCl3)Source of chirality: l-aspartic acid and asymmetric dihydroxylationAbsolute configuration : (2S,3S,5S)
(2R,3S,5S)-5-(((tert-Butyldiphenylsilyl)oxy)methyl)-2-(hydroxymethyl)pyrrolidin-3-olC22H31NO3Si[α]D25=-129.8 (c 0.02, CHCl3)Source of chirality: l-aspartic acid and asymmetric dihydroxylationAbsolute configuration : (2R,3S,5S)
(2R,3S,5S)-1-Benzyl-3-(benzyloxy)-2-((benzyloxy)methyl)-5-(((tert-butyldiphenylsilyl)oxy)methyl)pyrrolidineC43H49NO3Si[α]D25=-7.2 (c 0.1, CHCl3)Source of chirality: l-aspartic acid and asymmetric dihydroxylationAbsolute configuration : (2R,3S,5S)
((2S,4S,5R)-1-Benzyl-4-(benzyloxy)-5-((benzyloxy)methyl)pyrrolidin-2-yl)methanolC27H31NO3[α]D25=-37.7 (c 0.76, CHCl3)Source of chirality: l-aspartic acid and asymmetric dihydroxylationAbsolute configuration : (2S,4S,5R)
Journal: Tetrahedron: Asymmetry - Volume 22, Issue 11, 15 June 2011, Pages 1234–1238