| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346844 | 1500336 | 2015 | 6 صفحه PDF | دانلود رایگان |
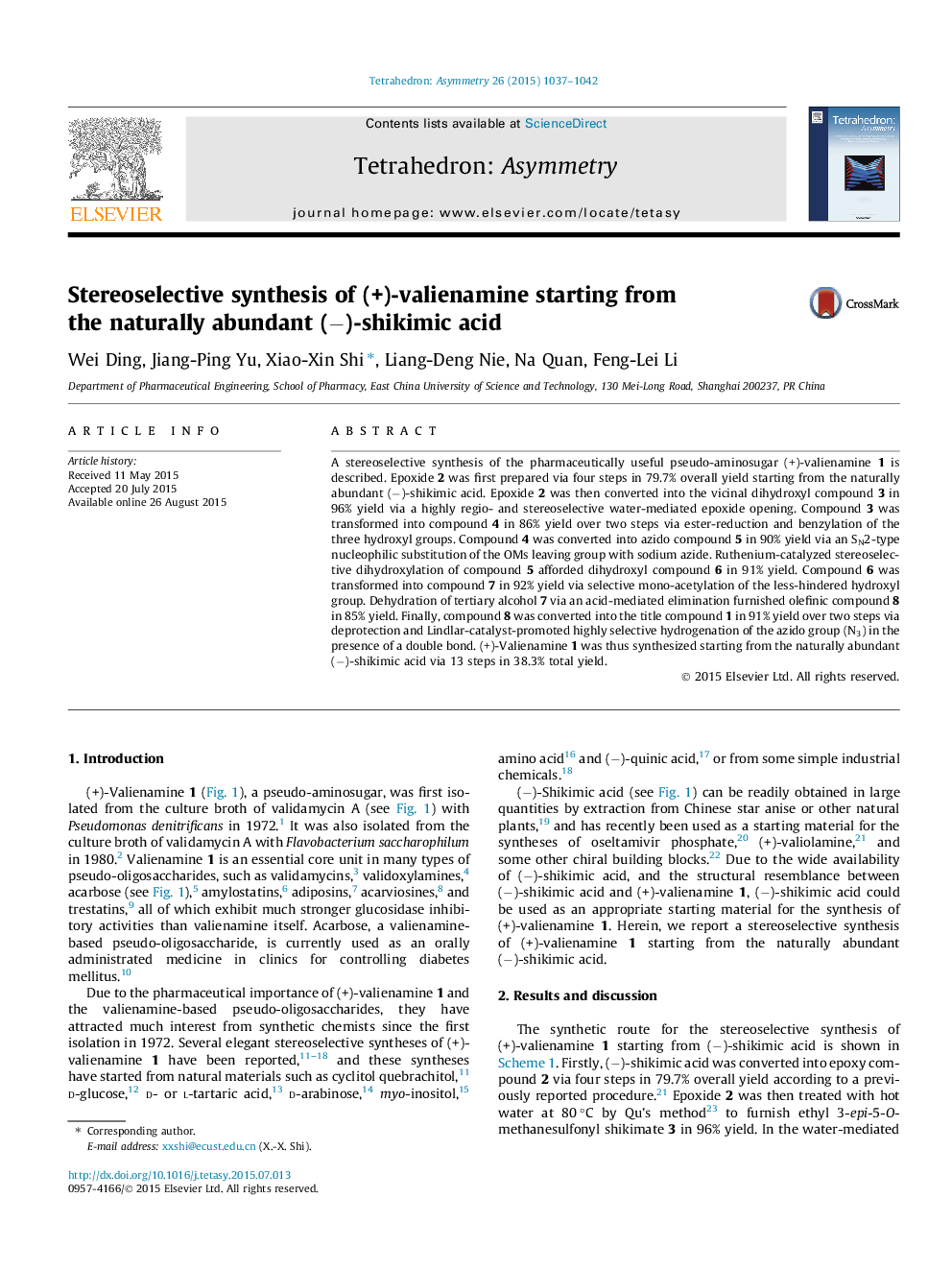
A stereoselective synthesis of the pharmaceutically useful pseudo-aminosugar (+)-valienamine 1 is described. Epoxide 2 was first prepared via four steps in 79.7% overall yield starting from the naturally abundant (−)-shikimic acid. Epoxide 2 was then converted into the vicinal dihydroxyl compound 3 in 96% yield via a highly regio- and stereoselective water-mediated epoxide opening. Compound 3 was transformed into compound 4 in 86% yield over two steps via ester-reduction and benzylation of the three hydroxyl groups. Compound 4 was converted into azido compound 5 in 90% yield via an SN2-type nucleophilic substitution of the OMs leaving group with sodium azide. Ruthenium-catalyzed stereoselective dihydroxylation of compound 5 afforded dihydroxyl compound 6 in 91% yield. Compound 6 was transformed into compound 7 in 92% yield via selective mono-acetylation of the less-hindered hydroxyl group. Dehydration of tertiary alcohol 7 via an acid-mediated elimination furnished olefinic compound 8 in 85% yield. Finally, compound 8 was converted into the title compound 1 in 91% yield over two steps via deprotection and Lindlar-catalyst-promoted highly selective hydrogenation of the azido group (N3) in the presence of a double bond. (+)-Valienamine 1 was thus synthesized starting from the naturally abundant (−)-shikimic acid via 13 steps in 38.3% total yield.
Figure optionsDownload as PowerPoint slide
(1S,2S,3S,4R)-1-Amino-5-hydroxymethyl-2,3,4-trihydroxycyclohex-5-ene [(+)-valienamine]C7H13NO4[α]D20 = +88.9 (c 0.35, H2O)Source of chirality: (−)-Shikimic acidAbsolute configuration: (1S,2S,3S,4R)
(3S,4R,5R)-Ethyl 5-O-(methanesulfonyl)shikimateC10H16O7S[α]D20 = −34.1 (c 1.00, CH3OH)Source of chirality: (−)-Shikimic acidAbsolute configuration: (3S,4R,5R)
(3S,4R,5R)-1-Benzoyloxymethyl-3,4-dibenzoyloxy-5-methanesulfonyloxy-cyclohex-1-eneC29H26O9S[α]D20 = +85.1 (c 0.80, CHCl3)Source of chirality: (−)-Shikimic acidAbsolute configuration: (3S,4R,5R)
(3S,4S,5S)-5-Azido-1-benzoyloxymethyl-3,4-dibenzoyloxycyclohex-1-eneC28H23N3O6[α]D20 = +76.6 (c 1.30, CHCl3)Source of chirality: (−)-Shikimic acidAbsolute configuration: (3S,4S,5S)
(1S,2S,3S,4S,5S)-1-Azido-5-benzoyloxymethyl-2,3-dibenzoyloxy-4,5-dihydroxycyclohexaneC28H25N3O8[α]D20 = −7.65 (c 1.00, CH3OH)Source of chirality: (−)-Shikimic acidAbsolute configuration: (1S,2S,3S,4S,5S)
(1S,2S,3R,4S,5S)-4-Acetoxy-1-azido-5-benzoyloxymethyl-2,3-dibenzoyloxy-5-hydroxycyclohexaneC30H27N3O9[α]D20 = +21.2 (c 1.00, CH3OH)Source of chirality: (−)-Shikimic acidAbsolute configuration: (1S,2S,3R,4S,5S)
(1S,2S,3S,4R)-4-Acetoxy-1-azido-5-benzoyloxymethyl-2,3-dibenzoyloxycyclohex-5-eneC30H25N3O8[α]D20 = +71.8 (c 1.00, CH3OH)Source of chirality: (−)-Shikimic acidAbsolute configuration: (1S,2S,3S,4R)
Journal: Tetrahedron: Asymmetry - Volume 26, Issues 18–19, 15 October 2015, Pages 1037–1042