| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346870 | 980283 | 2009 | 5 صفحه PDF | دانلود رایگان |
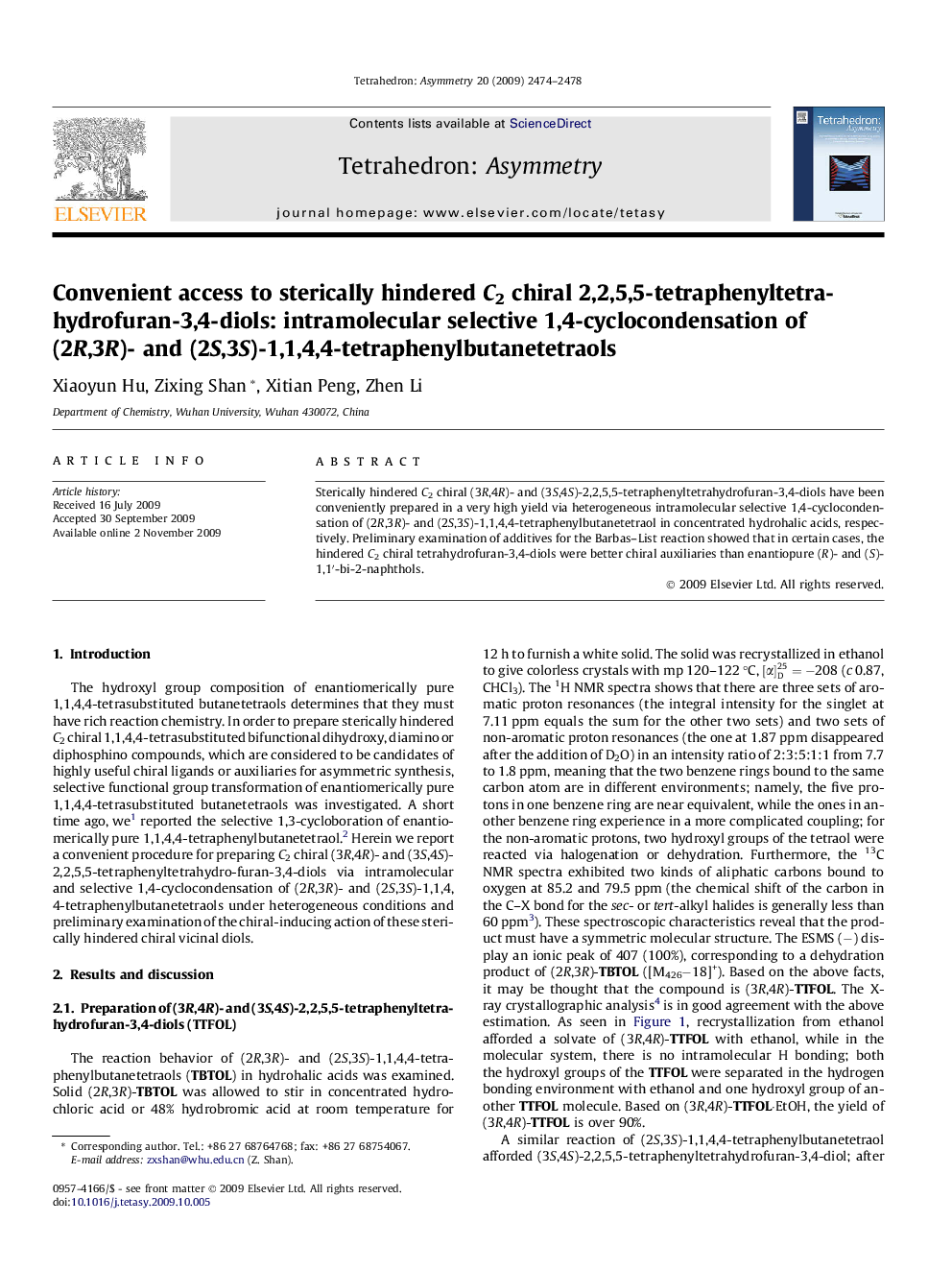
Sterically hindered C2 chiral (3R,4R)- and (3S,4S)-2,2,5,5-tetraphenyltetrahydrofuran-3,4-diols have been conveniently prepared in a very high yield via heterogeneous intramolecular selective 1,4-cyclocondensation of (2R,3R)- and (2S,3S)-1,1,4,4-tetraphenylbutanetetraol in concentrated hydrohalic acids, respectively. Preliminary examination of additives for the Barbas–List reaction showed that in certain cases, the hindered C2 chiral tetrahydrofuran-3,4-diols were better chiral auxiliaries than enantiopure (R)- and (S)-1,1′-bi-2-naphthols.
Figure optionsDownload as PowerPoint slide
(3R,4R)-2,2,5,5-Tetraphenyltetrahydrofuran-3,4-diolC28H24O3·C2H5OH[α]D27=-208 (c 0.87, CHCl3)Source of chirality: (2R,3R)-tartaric acidAbsolute configuration: (3R,4R)
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 21, 4 November 2009, Pages 2474–2478