| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346902 | 980284 | 2011 | 5 صفحه PDF | دانلود رایگان |
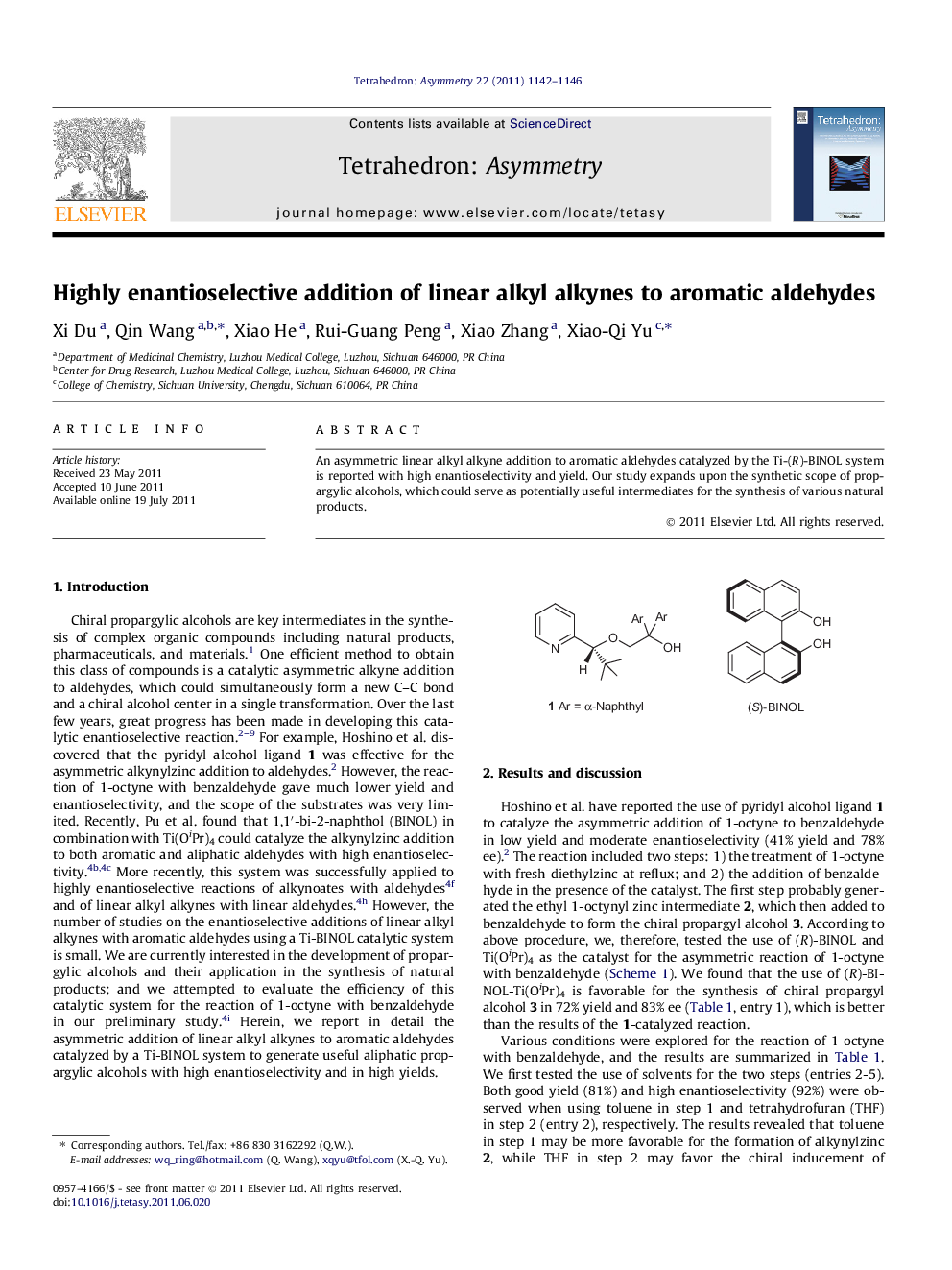
An asymmetric linear alkyl alkyne addition to aromatic aldehydes catalyzed by the Ti-(R)-BINOL system is reported with high enantioselectivity and yield. Our study expands upon the synthetic scope of propargylic alcohols, which could serve as potentially useful intermediates for the synthesis of various natural products.
Figure optionsDownload as PowerPoint slide
(S)-1-Phenyl-non-2-yn-1-olC15H20O[α]D20=-100.4 (c 0.91, CHCl3)Source of chirality: (R)-1, 1′-bi-2-naphtholAbsolute configuration: (S)
(S)-1-(2-Methylphenyl)-non-2-yn-1-olC16H22O[α]D20=-29.4 (c 0.50, CHCl3)Source of chirality: (R)-1, 1′-bi-2-naphtholAbsolute configuration: (S)
(S)-1-(3-Methylphenyl)-non-2-yn-1-olC16H22O[α]D20=-14.9 (c 0.50, CHCl3)Source of chirality: (R)-1,1′-bi-2-naphtholAbsolute configuration: (S)
(S)-1-(4-Methylphenyl)-non-2-yn-1-olC16H22O[α]D20=-23.0 (c 0.50, CHCl3)Source of chirality: (R)-1, 1′-bi-2-naphtholAbsolute configuration: (S)
(S)-1-(3-Methoxyphenyl)-non-2-yn-1-olC16H22O2[α]D20=-56.1 (c 0.50, CHCl3)Source of chirality: (R)-1, 1′-bi-2-naphtholAbsolute configuration: (S)
(S)-1-(4-Methoxyphenyl)-non-2-yn-1-olC16H22O2[α]D20=-59.7 (c 0.50, CHCl3)Source of chirality: (R)-1, 1′-bi-2-naphtholAbsolute configuration: (S)
(S)-1-(3-Fluorophenyl)-non-2-yn-1-olC15H19FO[α]D20=-54.4 (c 0.88, CHCl3)Source of chirality: (R)-1, 1′-bi-2-naphtholAbsolute configuration: (S)
(S)-1-(3-Chlorophenyl)-non-2-yn-1-olC15H19OCl[α]D20=-73.6 (c 1.00, CHCl3)Source of chirality: (R)-1, 1′-bi-2-naphtholAbsolute configuration: (S)
(S)-1-(3-Bromophenyl)-non-2-yn-1-olC15H19OBr[α]D20=-44.3 (c 0.45, CHCl3)Source of chirality: (R)-1, 1′-bi-2-naphtholAbsolute configuration: (S)
(S)-1-(2-Chlorophenyl)-non-2-yn-1-olC15H19OCl[α]D20=+25.5 (c 0.95, CHCl3)Source of chirality: (R)-1, 1′-bi-2-naphtholAbsolute configuration: (S)
(S)-1-(4-Fluorophenyl)-non-2-yn-1-olC15H19OF[α]D20=-18.0 (c 0.52, CHCl3)Source of chirality: (R)-1, 1′-bi-2-naphtholAbsolute configuration: (S)
(S)-1-Phenyl-hept-2-yn-1-olC13H16O[α]D20=-54.1 (c 0.79, CHCl3)Source of chirality: (R)-1, 1′-bi-2-naphtholAbsolute configuration: (S)
(S)-1-(4-Methylphenyl)-hept-2-yn-1-olC14H18O[α]D20=-67.4 (c 1.05, CHCl3)Source of chirality: (R)-1, 1′-bi-2-naphtholAbsolute configuration: (S)
(S)-1-(3-Methoxyphenyl)-hept-2-yn-1-olC14H18O2[α]D20=-63.2 (c 1.02, CHCl3)Source of chirality: (R)-1, 1′-bi-2-naphtholAbsolute configuration: (S)
(S)-1-(3-Fluorophenyl)-hept-2-yn-1-olC13H15FO[α]D20=-49.0 (c 0.97, CHCl3)Source of chirality: (R)-1, 1′-bi-2-naphtholAbsolute configuration: (S)
Journal: Tetrahedron: Asymmetry - Volume 22, Issue 10, 31 May 2011, Pages 1142–1146