| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346941 | 980286 | 2009 | 4 صفحه PDF | دانلود رایگان |
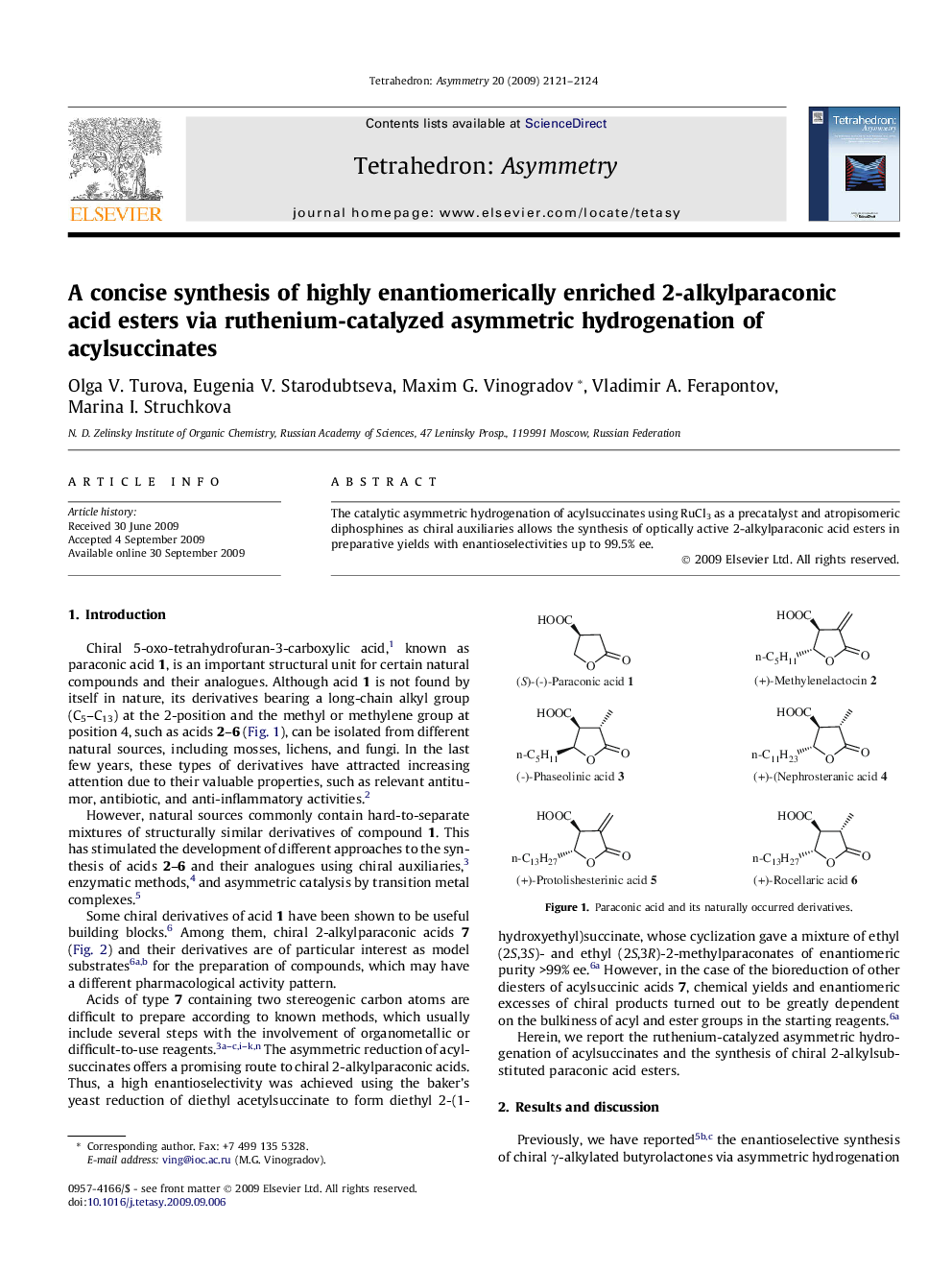
The catalytic asymmetric hydrogenation of acylsuccinates using RuCl3 as a precatalyst and atropisomeric diphosphines as chiral auxiliaries allows the synthesis of optically active 2-alkylparaconic acid esters in preparative yields with enantioselectivities up to 99.5% ee.
Figure optionsDownload as PowerPoint slide
Methyl (2R,3S)-2-methyl-5-oxo-tetrahydrofuran-3-carboxylateС7H10O4Ee = 89.5%[α]D20=+32.9 (c 0.23 CH3CN)[α]D20=+33.0 (c 0.216 CH2Cl2)Source of chirality: asymmetric catalysisAbsolute configuration: (2R,3S)
Methyl (2R,3R)-2-methyl-5-oxo-tetrahydrofuran-3-carboxylateС7H10O4Ee = 98.0%[α]D20=+83.2 (c 0.194 CH3CN)[α]D20=+79.9 (c 0.158 CH2Cl2)Source of chirality: asymmetric catalysisAbsolute configuration: (2R,3R)
Methyl (2R,3S)-2-ethyl-5-oxo-tetrahydrofuran-3-carboxylateС8H12O4Ee = 94.0%[α]D20=+38.2 (c 0.202 CH3CN)[α]D20=+39.8 (c 0.170 CH2Cl2)Source of chirality: asymmetric catalysisAbsolute configuration: (2R,3S)
Methyl (2R,3R)-2-ethyl-5-oxo-tetrahydro-3-furancarboxylateС8H12O4Ee = 99.0%[α]D20=+104.4 (c 0.198 CH3CN)[α]D20=+105.4 (c 0.234 CH2Cl2)Source of chirality: asymmetric catalysisAbsolute configuration: (2R,3R)
Methyl (2R,3S)-2-propyl-5-oxo-tetrahydrofuran-3-carboxylateС9H14O4Ee = 96.0%[α]D20=+39.4 (c 0.164 CH3CN)[α]D20=+39.3 (c 0.186 CH2Cl2)Source of chirality: asymmetric catalysisAbsolute configuration: (2R,3S)
Methyl (2R,3R)-2-propyl-5-oxo-tetrahydrofuran-3-carboxylateС9H14O4Ee = 98.5%[α]D20=+93.2 (c 0.160 CH3CN)[α]D20=+94.0 (c 0.328 CH2Cl2)Source of chirality: asymmetric catalysisAbsolute configuration: (2R,3R)
Methyl (2R,3S)-2-iso-propyl-5-oxo-tetrahydrofuran-3-carboxylateС9H14O4Ee = 84.0%[α]D20=+24.9 (c 0.318 CH3CN)[α]D20=+23.9 (c 0.304 CH2Cl2)Source of chirality: asymmetric catalysisAbsolute configuration: (2R,3S)
Methyl (2R,3R)-2-iso-propyl-5-oxo-tetrahydrofuran-3-carboxylateС9H14O4Ee = 99.5%[α]D20=+38.0 (c 0.108 CH2Cl2)Source of chirality: asymmetric catalysisAbsolute configuration: (2R,3R)
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 18, 23 September 2009, Pages 2121–2124