| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346944 | 980286 | 2009 | 8 صفحه PDF | دانلود رایگان |
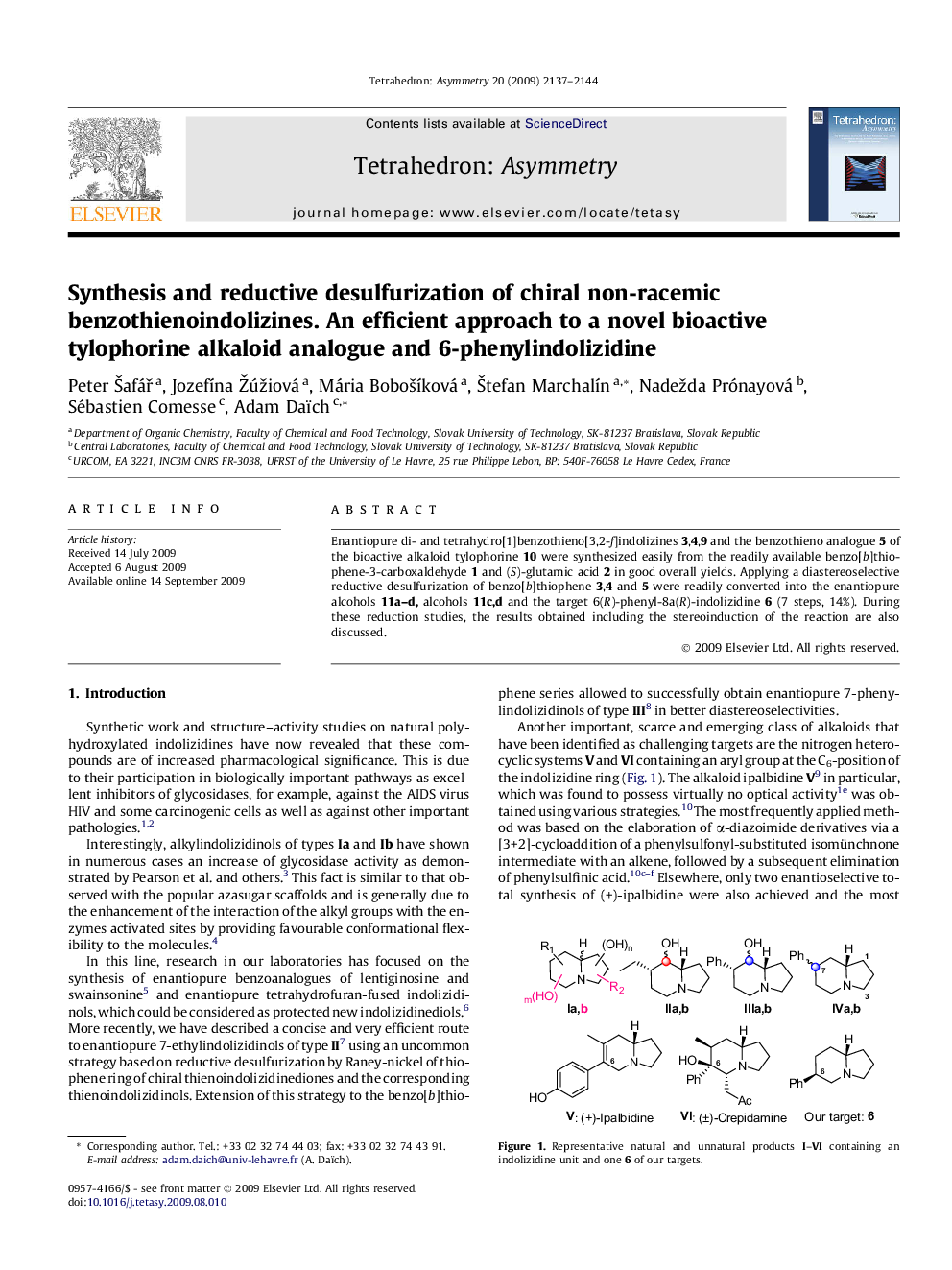
Enantiopure di- and tetrahydro[1]benzothieno[3,2-f]indolizines 3,4,9 and the benzothieno analogue 5 of the bioactive alkaloid tylophorine 10 were synthesized easily from the readily available benzo[b]thiophene-3-carboxaldehyde 1 and (S)-glutamic acid 2 in good overall yields. Applying a diastereoselective reductive desulfurization of benzo[b]thiophene 3,4 and 5 were readily converted into the enantiopure alcohols 11a–d, alcohols 11c,d and the target 6(R)-phenyl-8a(R)-indolizidine 6 (7 steps, 14%). During these reduction studies, the results obtained including the stereoinduction of the reaction are also discussed.
Figure optionsDownload as PowerPoint slide
(S)-1-(Benzo[b]thien-3-ylmethyl)-5-oxopyrrolidine-2-carboxylic acidC14H13NO3SAbsolute configuration: (S)[α]D20=+42.4 (c 1.0, MeOH)Source of chirality: (S)-glutamic acid
(11aS)-1,11a-Dihydro[1]benzothieno[3,2-f]indolizine-3,11(2H,5H)-dioneC14H11NO2SAbsolute configuration: (S)[α]D20=+73.6 (c 1.0, MeOH)Source of chirality: (S)-glutamic acid
(11S,11aS)-11-Hydroxy-1,5,11,11a-tetrahydro[1]benzothieno[3,2-f]indolizin-3(2H)-oneC14H13NO2SAbsolute configuration: (11S,11aS)[α]D20=+128.6 (c 1.0, MeOH)Source of chirality: (S)-glutamic acid
(11aS)-1,5,11,11a-Tetrahydro[1]benzothieno[3,2-f]indolizin-3(2H)-oneC14H13NOSAbsolute configuration: (S)[α]D20=+285.1 (c 1.0, MeOH)Source of chirality: (S)-glutamic acid
(11aS)-1,2,3,5,11,11a-Hexahydro[1]benzothieno[3,2-f]indolizineC14H15NSAbsolute configuration: (S)[α]D20=+166.0 (c 1.0, MeOH)Source of chirality: (S)-glutamic acid
(6R,8R,8aS)-8-Hydroxy-6-phenylhexahydroindolizin-3(5H)-oneC14H17NO2Absolute configuration: (6R,8R,8aS)[α]D20=+14.2 (c 1.0, MeOH)Source of chirality: (S)-glutamic acid
(6R,8aR)-6-PhenyloctahydroindolizineC14H19NAbsolute configuration: (6R,8aR)[α]D20=-2.0 (c 1.0, MeOH)Source of chirality: (S)-glutamic acid
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 18, 23 September 2009, Pages 2137–2144