| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346962 | 980288 | 2009 | 6 صفحه PDF | دانلود رایگان |
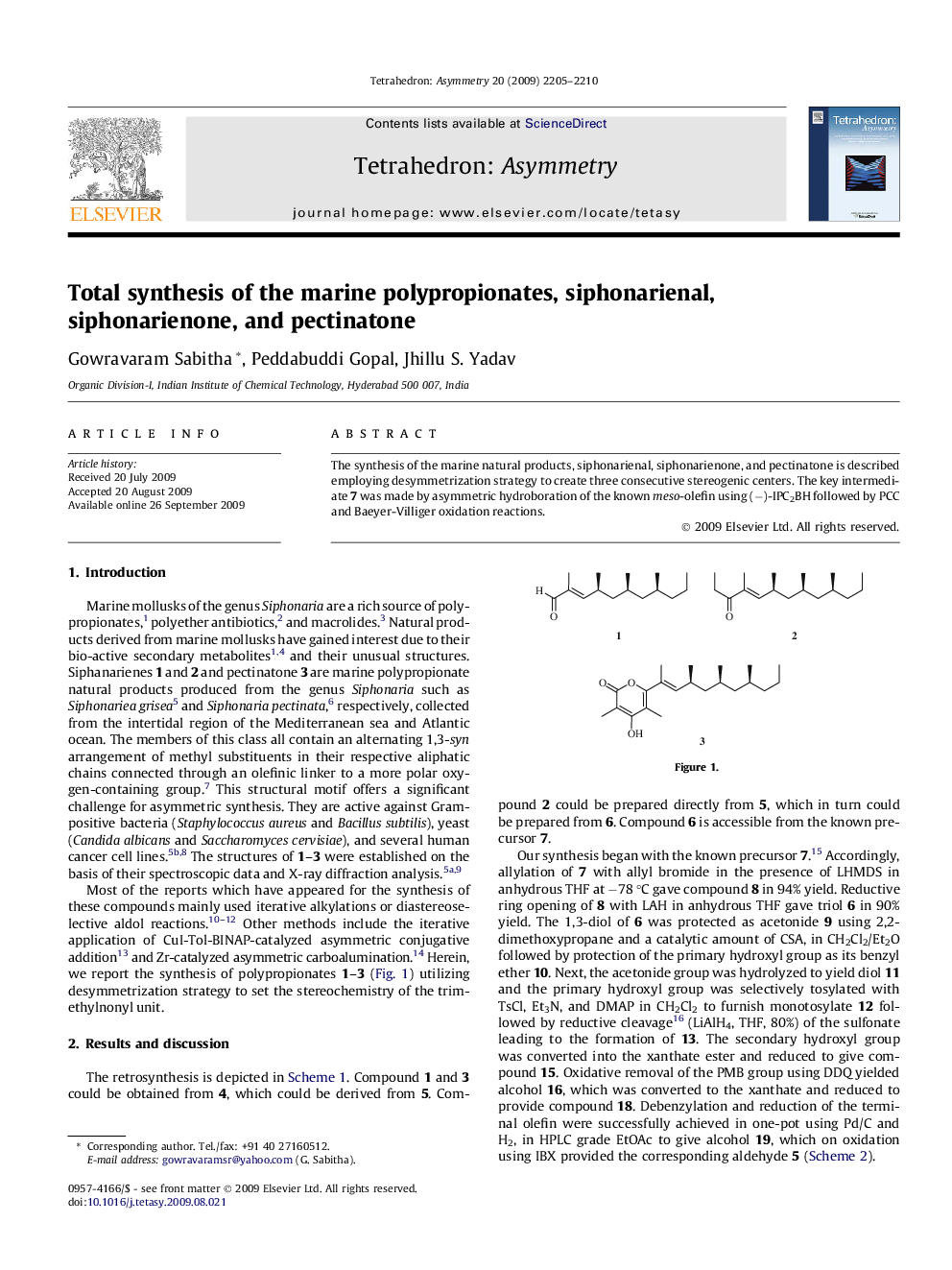
The synthesis of the marine natural products, siphonarienal, siphonarienone, and pectinatone is described employing desymmetrization strategy to create three consecutive stereogenic centers. The key intermediate 7 was made by asymmetric hydroboration of the known meso-olefin using (−)-IPC2BH followed by PCC and Baeyer-Villiger oxidation reactions.
Figure optionsDownload as PowerPoint slide
(E,4S,6S,8S)-2,4,6,8-Tetramethyl-2-undecenalC15H28O[α]D25=+16.5 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (E,4S,6S,8S)
Siphonarienone, [(E,6S,8S,10S)-4,6,8,10-tetramethyl-4-tridecen-3-one]C17H32O[α]D25=+16.0 (c 1.2, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (E,6S,8S,10S)
Pectinatone, [4-hydroxy-3,5-dimethyl-6-[(E,3S,5S,7S)-1,3,5,7-tetramethyl-1-decenyl]-2H-2-pyranone]C21H34O3[α]D25=+60.5 (c 0.1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (E,3S,5S,7S)
Ethyl (E,4S,6S,8S)-2,4,6,8-tetramethyl-2-undecenoateC17H32O2[α]D25=+26.5 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (E,4S,6S,8S)
(2S,4S,6S)-2,4,6-TrimethylnonanalC12H24O[α]D25=+6.2 (c 0.26, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3R,4R,5S,9S)
(2R,3R,4S,5R,6R)-2-Allyl-5-[(4-methoxybenzyl)oxy]-4,6-dimethylheptane-1,3,7-triolC20H32O5[α]D25=+6.0 (c 1.4, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2R,3R,4S,5R,6R)
7-(4-Methoxybenzyloxy)-4(2′-propen),6,8-diimethyl-(1S,4S,5S,6S,7S,8R)-2,9-dioxabicyclo-[3.3.1]nonan-3-oneC20H26O5[α]D25=-75 (c 1.3, CHCl3)Source of Chirality: Asymmetric SynthesisAbsolute configuration: (1S,4S,5S,6S,7S,8R)
(2R,3R,4R)-4-[(4R,5R)-5-Allyl-2,2-dimethyl-1,3-dioxan-4-yl]-3-[(4-methoxybenzyl)oxy]-2-methylpentan-1-olC23H36O5[α]D25=-33.0 (c 1.1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2R,3R,4R, 4R,5R)
(4R,5R)-5-Allyl-4-(1R,2R,3R)-4-(benzyloxy)-2-[(4-methoxybenzyl)oxy]-1,3-dimethylbutyl-2,2-dimethyl-1,3-dioxaneC30H42O5[α]D25=-18.0 (c 1.1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (4R,5R, 1R,2R,3R)
(2R,3R,4S,5R,6R)-2-Allyl-7-(benzyloxy)-5-[(4-methoxybenzyl)oxy]-4,6-dimethylheptane-1,3-diolC27H38O5[α]D25=+18.0 (c 1.15, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2R,3R,4S,5R,6R)
(4S,5R,6S,7R,8R)-9-(Benzyloxy)-7-[(4-methoxybenzyl)oxy]-4,6,8-trimethyl-1-nonen-5-olC27H38O4[α]D25=+26.6 (c 1.15, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (4S,5R,6S,7R,8R)
(1R,2S)-1-(1R,2R,3R)-4-(Benzyloxy)-2-[(4-methoxybenzyl)oxy]-1,3-dimethylbutyl-2-methyl-4-pentenyl (methylsulfanyl)methanethioateC29H40O4S2[α]D25=+2.3 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (1R,2S,1R,2R,3R)
(4S,6S,7S,8R)-9-(Benzyloxy)-7-[(4-methoxybenzyl)oxy]-4,6,8-trimethyl-1-noneneC27H38O3[α]D25=+12.9 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (4S,6S,7S,8R)
(2R,3S,4S,6S)-1-(Benzyloxy)-2,4,6-trimethyl-8-nonen-3-olC19H30O2[α]D25=-25.0 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2R,3S,4S,6S)
(1S,2S,4S)-1-[(1R)-2-(Benzyloxy)-1-methylethyl]-2,4-dimethyl-6-heptenyl methylsulfanyl)methanethioateC21H32O2S2[α]D25=+11.5 (c 1.1, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (1S,2S,4S,1R)
(4S,6S,8S)-9-(Benzyloxy)-4,6,8-trimethyl-1-noneneC19H30O[α]D25=+2.9 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (4S,6S,8S)
(2S,4S,6S)-2,4,6-Trimethylnonan-1-olC12H26O[α]D25=+5.2 (c 0.3, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,4S,6S)
(E,4S,6S,8S)-2,4,6,8-Tetramethyl-2-undecen-1-olC15H30O[α]D25=+9.4 (c 0.2, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (E,4S,6S,8S)
N-1-Methoxy-N-1,2,4,6,8-pentamethyl-(E,4S,6S,8S)-2-undecenamideC17H33NO2[α]D25=+12.0 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (E,4S,6S,8S)
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 19, 6 October 2009, Pages 2205–2210