| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1346967 | 980288 | 2009 | 4 صفحه PDF | دانلود رایگان |
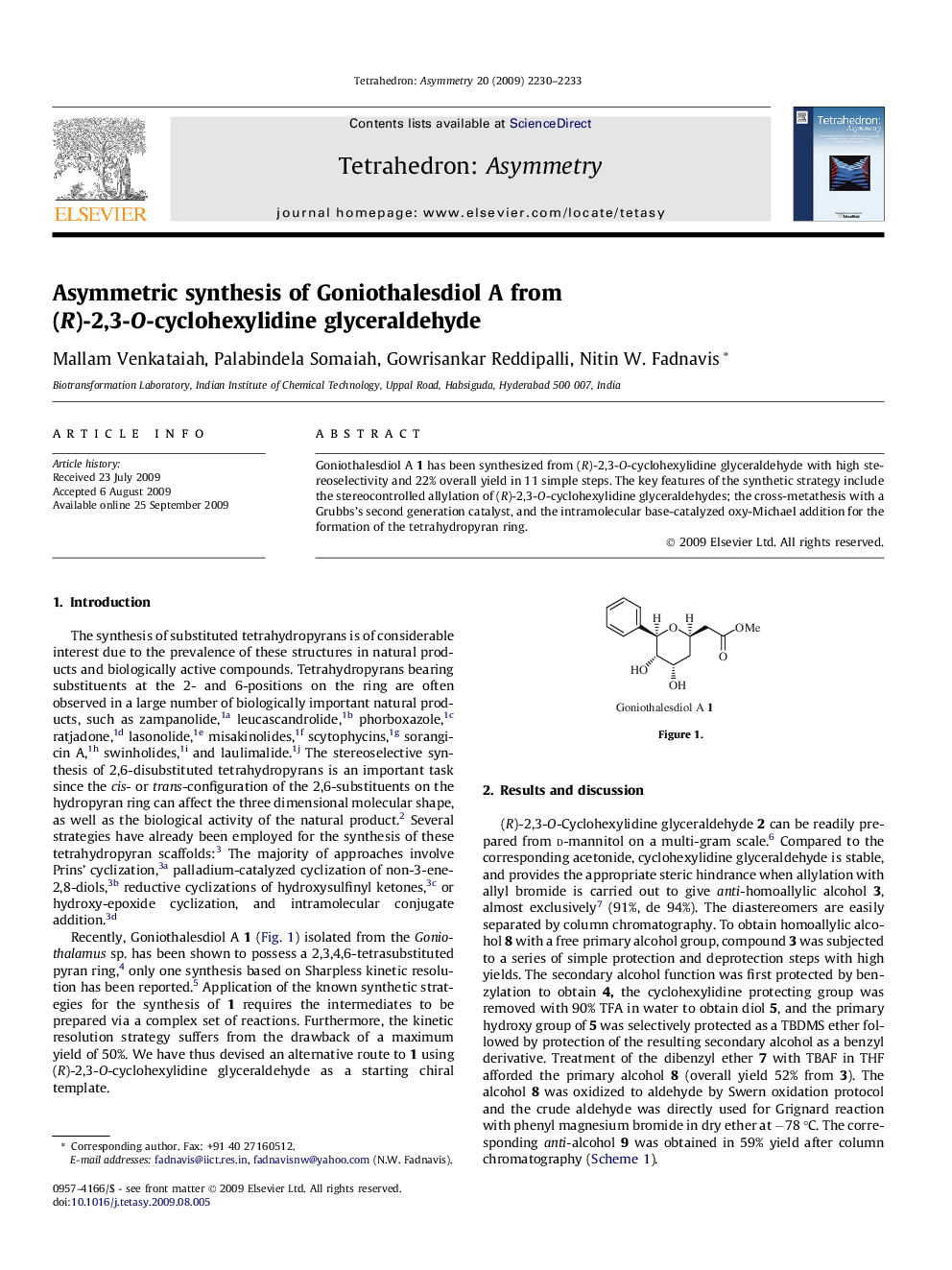
Goniothalesdiol A 1 has been synthesized from (R)-2,3-O-cyclohexylidine glyceraldehyde with high stereoselectivity and 22% overall yield in 11 simple steps. The key features of the synthetic strategy include the stereocontrolled allylation of (R)-2,3-O-cyclohexylidine glyceraldehydes; the cross-metathesis with a Grubbs’s second generation catalyst, and the intramolecular base-catalyzed oxy-Michael addition for the formation of the tetrahydropyran ring.
Figure optionsDownload as PowerPoint slide
(S)-1-((R)-1,4-Dioxaspiro[4.5]decan-2-yl)but-3-en-1-olC12H20O3[α]D25=+10.0 (c 1, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (1S,2R)
(R)-2-((S)-1-(Benzyloxy)but-3-enyl)-1,4-dioxaspiro[4.5]decane(R)-2-((S)-1-(benzyloxy)but-3-enyl)-1,4-dioxaspiro[4.5]decaneC19H26O3[α]D25=+27.0 (c 1, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (1S,2R)
(R)-2-((S)-1-(Benzyloxy)but-3-enyl)-1,4-dioxaspiro[4.5]decane(2R,3S)-3-(benzyloxy)hex-5-ene-1,2-diolC13H18O3[α]D25=+34.0 (c 1, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (2R,3S)
(R)-2-((S)-1-(Benzyloxy)but-3-enyl)-1,4-dioxaspiro[4.5]decane(2R,3S)-3-(benzyloxy)-1-(tert butyldimethylsilyloxy)hex-5-en-2-olC19H32O3[α]D25=+26.0 (c 1, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (2R,3S)
(R)-2-((S)-1-(Benzyloxy)but-3-enyl)-1,4-dioxaspiro[4.5]decane(2R,3S)-2,3-bis(benzyloxy)hex-5-en-1-olC20H24O3[α]D25=+9.5 (c 1, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (2R,3S)
(R)-2-((S)-1-(Benzyloxy)but-3-enyl)-1,4-dioxaspiro[4.5]decane(1R,2R,3S)-2,3-bis(benzyloxy)-1-phenylhex-5-en-1-olC26H28O3[α]D25=+40.0 (c 1, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (1R,2R,3S)
(R)-2-((S)-1-(Benzyloxy)but-3-enyl)-1,4-dioxaspiro[4.5]decane(5S,6R,7R,E)-methyl 5,6-bis(benzyloxy)-7-hydroxy-7-phenylhept-2-enoateC28H30O5[α]D25=+11.0 (c 1, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (5S,6R,7R)
Methyl 2-((2R,4S,5S,6R)-4,5-bis(benzyloxy)-6-phenyltetrahydro-2H-pyran-2-yl)acetatemethyl 2-((2R,4S,5S,6R)-4,5-bis(benzyloxy)-6-phenyltetrahydro-2H-pyran-2-yl)acetateC28H30O5[α]D25=+28.5 (c 1, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (2R,4S,5S,6R)
Methyl 2-((2R,4S,5S,6R)-4,5-bis(benzyloxy)-6-phenyltetrahydro-2H-pyran-2-yl)acetatemethyl 2-((2R,4S,5S,6R)-4,5-dihydroxy-6-phenyltetrahydro-2H-pyran-2-yl)acetateC14H18O5[α]D25=-28.0 (c 0.5, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (2R,4S,5S,6R)
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 19, 6 October 2009, Pages 2230–2233