| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347024 | 980291 | 2009 | 6 صفحه PDF | دانلود رایگان |
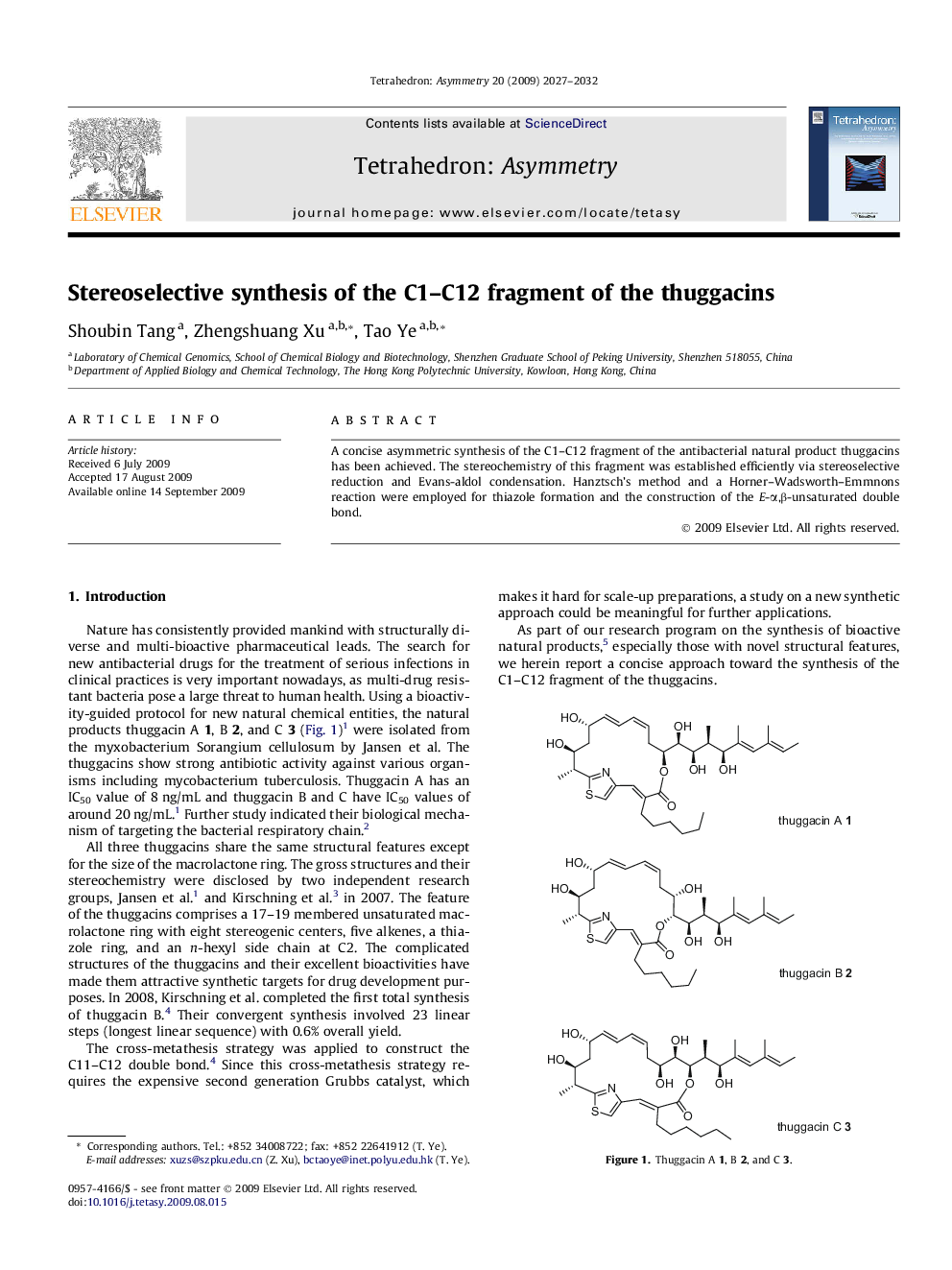
A concise asymmetric synthesis of the C1–C12 fragment of the antibacterial natural product thuggacins has been achieved. The stereochemistry of this fragment was established efficiently via stereoselective reduction and Evans-aldol condensation. Hanztsch’s method and a Horner–Wadsworth–Emmnons reaction were employed for thiazole formation and the construction of the E-α,β-unsaturated double bond.
Figure optionsDownload as PowerPoint slide
(2R,3S,5S)-3,5-Di-(tert-butyldimethylsilyloxy)-2-methyl-7-trimethylsilyl-hept-6-ynic-acidC23H48O4Si3De = 100%[α]D20=-28.8 (c 1.2, CHCl3)Source of chirality: reaction substrateAbsolute configuration: (2R,3S,5S)
(2R,3S,5S)-3,5-Di-(tert-butyldimethylsilyloxy)-2-methyl-7-trimethylsilyl-6-heptynamideC23H49NO3Si3De = 100%[α]D20=-34.5 (c 3.3, CHCl3)Source of chirality: reaction substrateAbsolute configuration: (2R,3S,5S)
(2R,3S,5S)-3,5-Di-(tert-butyldimethylsilyloxy)-2-methyl-7-trimethylsilyl-6-heptyne-thioamideC23H49NO2Si3SDe = 100%[α]D20=-26.8 (c 0.85, CHCl3)Source of chirality: reaction substrateAbsolute configuration: (2R,3S,5S)
2-[(1′R,2′S,4′S)-2′,4′-Bis-(tert-butyl-dimethyl-silanyloxy)-1′-methyl-6′-trimethylsilanyl-hex-5′-ynyl]-thiazole-4-carboxylic acid ethyl esterC28H53NO4Si3SDe = 100%[α]D20=-18.4 (c 1.05, CHCl3)Source of chirality: reaction substrateAbsolute configuration: (1′R, 2′S, 4′S)
2-[(1′R,2′S,4′S)-2′,4′-Di(tert-butyldimethylsilyloxy)-1′-methyl-5′-hexynyl]-4-thiazole carboxylic acid, ethyl esterC25H45NO4Si2SDe = 100%[α]D20=-21.4 (c 1.05, CHCl3)Source of chirality: reaction substrateAbsolute configuration: (1′R, 2′S, 4′S)
2-[(1′R,2′S,4′S)-2′,4′-Di-(tert-butyldimethylsilyloxy)-6′-iodo-1′-methyl-(E)-5′-hexenyl]-4-thiazole carboxylic acid, ethyl esterC25H46INO4Si2SDe = 100%[α]D20=-27.2 (c 1.4, CHCl3)Source of chirality: E-vinyl iodination and reaction substrateAbsolute configuration: (1′R, 2′S, 4′S)
2-[(1′R,2′S,4′S)-2′,4′-Di-(tert-butyldimethylsilyloxy)-6′-iodo-1′-methyl-(E)-5′-hexenyl]-4-formylthiazoleC23H42INO3Si2SDe = 100%[α]D20=-14.3 (c 1.1, CHCl3)Source of chirality: reaction substrateAbsolute configuration: (1′R, 2′S, 4′S)
3-{2′-[(1″R,2″S,4″S)-2″,4″-Bis-(tert-butyl-dimethyl-silanyloxy)-6″-iodo-1″-methyl-hex-5″-enyl]-thiazol-4′-yl}-2-hexyl-acrylic acid ethyl esterC33H60INO4Si2SDe = 100%[α]D20=-36.3 (c 1.2, CHCl3)Source of chirality: HWE olefination and reaction substrateAbsolute configuration: (1″R, 2″S, 4″S)
3S-5-(4-Methoxybenzyloxy)-1-trimethylsilanyl-pent-1-yn-3-olC16H24O3SiEe = 82%[α]D20=-13.7 (c 2.4, CHCl3)Source of chirality: asymmetric reduction of ketone with S-alpineAbsolute configuration: (3S)
3S-3-(tert-Butyl-dimethyl-silanyloxy)-5-trimethylsilanyl-pent-4-yn-1-olC14H30O2Si2Ee = 82%[α]D20=-9.8 (c 1.4, CHCl3)Source of chirality: reaction substrateAbsolute configuration: (3S)
3S-3-(tert-Butyl-dimethyl-silanyloxy)-5-trimethylsilanyl-pent-4-ynalC14H28O2Si2Ee = 82%[α]D20=-13.2 (c 2.1, CHCl3)Source of chirality: reaction substrateAbsolute configuration: (3S)
(2′R, 3′S, 5′R)-4-Benzyl-3-[5′-(tert-butyldimethyl-silanyloxy)-3′-hydroxy-2′-methyl-7′-trimethylsilanyl-hept-6′-ynoyl]-oxazolidin-2-oneC27H43NO5Si2De = 92%[α]D20=-44.3 (c 1.5, CHCl3)Source of chirality: asymmetric Evans-aldol reactionAbsolute configuration: (2′R, 3′S, 5′R)
(2′R, 3′S, 5′R)-4-Benzyl-3-[3′,5′-bis-(tert-butyldimethyl-silanyloxy)-2′-methyl-7′-trimethylsilanyl-hept-6′-ynoyl]-oxazolidin-2-oneC33H57NO5Si3De = 100%[α]D20=-38.4 (c 2.1, CHCl3)Source of chirality: reaction substrateAbsolute configuration: (2′R, 3′S, 5′R)-
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 17, 8 September 2009, Pages 2027–2032